7752-72-9
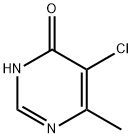 7752-72-9 结构式
7752-72-9 结构式
基本信息
5-氯-6-甲基嘧啶-4(1H)-酮
5-氯-6-甲基嘧啶-4(3H)-酮
5-氯-6-甲基-4(3H)-嘧啶酮
4-羟基-5-氯-6-甲基嘧啶 100MG
4-Chloro-6-methyl-pyrimidin-5-ol
5-Chloro-6-methyl-1H-pyrimidin-4-one
5-CHLORO-6-METHYLPYRIMIDIN-4(1H)-ONE
5-Chloro-6-MethylpyriMidin-4(3H)-one
5-Chloro-6-hydroxy-4-methylpyrimidine
4(3H)-Pyrimidinone, 5-chloro-6-methyl-
4(1H)-Pyrimidinone, 5-chloro-6-methyl- (9CI)
物理化学性质
制备方法
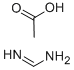
3473-63-0
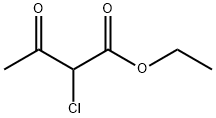
609-15-4

7752-72-9
以醋酸甲脒和2-氯乙酰乙酸乙酯为原料合成5-氯-6-甲基嘧啶-4(1H)-酮的一般步骤如下:将4-羟基-5-氯-6-甲基嘧啶(8.80g,0.16mol)的甲醇溶液缓慢加入到含有11.30g(0.11mol)醋酸甲脒的50mL甲醇溶液中,反应在室温下搅拌进行。加入完毕后,继续在室温下搅拌混合物2小时。随后,加入11.17g(0.068mol)2-氯-3-氧代丁酸乙酯,室温下继续搅拌反应5-7小时。反应进程通过薄层色谱(TLC)监测。反应完成后,将反应混合物减压浓缩,用盐酸(HCl)调节pH至5-6,过滤得到橙黄色固体。水相用乙酸乙酯(3×50mL)萃取,合并有机相,用无水硫酸镁干燥,过滤后减压浓缩。将残余物溶于50mL乙酸乙酯中,静置过夜,得到6.48g橙黄色固体产物,产率为66%,熔点为181~184°C。
参考文献:
[1] Patent: EP2913325, 2015, A1. Location in patent: Paragraph 0451; 0452
[2] Patent: CN105348298, 2016, A. Location in patent: Paragraph 0418; 0419; 0420
[3] Patent: CN105732585, 2016, A. Location in patent: Paragraph 0368; 0369; 0370
[4] Patent: CN105777717, 2016, A. Location in patent: Paragraph 0356; 0357; 0358; 0359
[5] Patent: CN104292169, 2018, B. Location in patent: Paragraph 0457; 0458; 0459
