785051-54-9
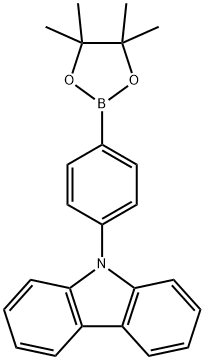 785051-54-9 结构式
785051-54-9 结构式
基本信息
9-(4-硼酸频哪醇酯苯基)咔唑
4-(咔唑-9基)苯硼酸频那醇酯
4-(9-咔唑基)苯硼酸频哪醇酯
9-[4-苯基频哪醇酯]-9H-咔唑
4-(9-咔唑基)苯硼酸频哪酯,95%
9H-咔唑-9-(4-苯基)硼酸频哪醇酯
9-[4-(4,4,5,5-四甲基-1,3,2-二噁硼烷-2-基)苯基]-9H-咔唑
9-[4-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)苯基]-9H-咔唑
9-[4-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)苯基]-9H-咔唑
2-dioxaborolan-2-yl)phenyl)-9H-carbazole
4-(9-Carbazolyl)phenylboronic Acid Pinacol Ester
4-(9-Carbazolyl)benzeneboronic acid pinacol ester
4-(9-Carbazolyl)benzeneboronicacidpinacolester,95%
9-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)
9H-Carbazole-9-(4-phenyl) boronic acid pinacol ester
9-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]carbazole
9-(4-(4,4,5,5-Tetramethy-1,3,2-dioxaborolan-2-yl)phenyl)-9H-carbazole
2-[4-(9H-Carbazol-9-yl)phenyl]-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
物理化学性质
制备方法
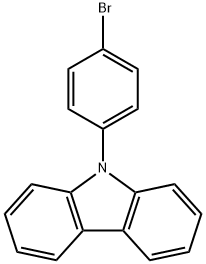
57102-42-8

73183-34-3

785051-54-9
以9-(4-溴苯基)咔唑和联硼酸频那醇酯为原料合成9-[4-(4,4,5,5-四甲基-1,3,2-二噁硼烷-2-基)苯基]-9H-咔唑的一般步骤:向100 mL圆底烧瓶中加入1 g 9-(4-溴苯基)咔唑(3.1 mmol)、0.79 g 联硼酸频那醇酯(3.1 mmol)、30 mL 1,4-二氧六环、0.4 g 乙酸钾(4 mmol)和0.13 g [1,1'-双(二苯基膦基)二茂铁]二氯化钯二氯甲烷络合物(0.16 mmol),在85℃下反应48小时。反应完成后,冷却至室温,加入200 mL 3M盐酸水溶液淬灭反应。分离有机层,用水洗涤。蒸发去除溶剂后,以二氯甲烷:石油醚=1:2(体积比)作为洗脱剂,通过柱色谱法纯化,得到1 g目标产物,产率为87%。
参考文献:
[1] Chemical Communications, 2012, vol. 48, # 71, p. 8970 - 8972
[2] Patent: CN108191739, 2018, A. Location in patent: Paragraph 0087; 0088; 0089
[3] Patent: EP1820801, 2007, A1. Location in patent: Page/Page column 88
[4] Organic Electronics: physics, materials, applications, 2014, vol. 15, # 7, p. 1413 - 1421
[5] Patent: WO2012/37269, 2012, A1. Location in patent: Page/Page column 45-46
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | T3452 | 9-[4-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)苯基]-9H-咔唑 9-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-9H-carbazole | 785051-54-9 | 1g | 150元 |
| 2025/12/22 | XW0278505154903 | 9-(4-(4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)苯基)-9H-咔唑 | 785051-54-9 | 10G | 193元 |
| 2025/12/22 | XW0278505154902 | 9-(4-(4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)苯基)-9H-咔唑 | 785051-54-9 | 5G | 97元 |
