81470-51-1
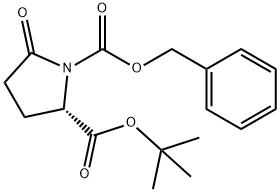 81470-51-1 结构式
81470-51-1 结构式
基本信息
1-苄基 2-叔丁基 (S)-5-氧代吡咯烷-1,2-二羧酸酯
TERT-BUTYL N-BENZYLOXYCARBONYL-L-PYROGLUTAMATE
tert-butylN-benzyloxycarbonyl-L-pyroglutamate
1-benzyl 2-tert-butyl (2S)-5-oxopyrrolidine-1,2-dicarboxylate
1-Benzyl 2-(2-methyl-2-propanyl) (2S)-5-oxo-1,2-pyrrolidinedicarboxylate
(2S)-5-Oxo-1,2-pyrrolidinedicarboxylic acid 2-(1,1-dimethylethyl) 1-(phenylmethyl) ester
1,2-Pyrrolidinedicarboxylic acid, 5-oxo-, 2-(1,1-dimethylethyl)1-(phenylmethyl) ester, (S)-
物理化学性质
制备方法
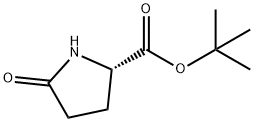
35418-16-7

501-53-1

81470-51-1
向(S)-5-氧代吡咯烷-2-羧酸叔丁酯(2.29g,12.4mmol)的四氢呋喃(THF,62mL)溶液中加入氢化钠(NaH,60%油分散体,546mg,13.6mmol,1.1当量),室温下搅拌30分钟。随后加入氯甲酸苄酯(1.94mL,13.6mmol,1.1当量),继续搅拌20小时。反应完成后,减压蒸发除去溶剂,加入饱和氯化铵(NH4Cl)水溶液(100mL),用乙酸乙酯(EtOAc,2×100mL)萃取。合并有机相,用硫酸镁(MgSO4)干燥,过滤后减压浓缩。通过硅胶(SiO2)柱色谱纯化,洗脱剂为环己烷/乙酸乙酯(3:1),得到1-苄基 2-叔丁基 (S)-5-氧代吡咯烷-1,2-二羧酸酯(3.56g,11.1mmol,收率90%),为无色油状物。薄层色谱(TLC)Rf值为0.18(环己烷/乙酸乙酯 3:1,高锰酸钾显色)。1H-NMR(300MHz,CDCl3)δ:1.39(s,9H,3×CH3),1.97-2.70(m,4H,2×CH2),4.54(dd,J=9.3,2.7Hz,1H,CH),5.27(dd,J=16.2,12.3Hz,2H,CH2),7.27-7.43(m,5H,Ar-H)。13C-NMR(75.5MHz,CDCl3)δ:25.11, 27.12, 31.23, 59.60, 68.43, 82.76, 128.4, 128.6, 128.7, 135.3, 151.1, 170.3, 173.3。
参考文献:
[1] Organic Letters, 2014, vol. 16, # 20, p. 5254 - 5257
[2] Patent: WO2015/110271, 2015, A1. Location in patent: Page/Page column 92; 95
[3] Patent: EP2899192, 2015, A1. Location in patent: Paragraph 0152; 0153
[4] Patent: US2017/2003, 2017, A1. Location in patent: Paragraph 0295-0298
[5] Patent: WO2004/50084, 2004, A2. Location in patent: Page/Page column 60; 61