82911-78-2
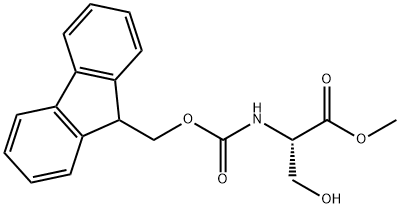 82911-78-2 结构式
82911-78-2 结构式
基本信息
N-芴甲氧羰基-L-丝氨酸甲酯
Fmoc-L-Ser-OMe
FMOC-SERINE-OME
FMOC-L-SERINE METHYL ESTER
Fmoc-L-β-hydroxyalanine methyl ester
Fmoc-L-serine methyl ester≥ 99.5% (HPLC)
(9H-Fluoren-9-yl)MethOxy]Carbonyl Ser-OMe
(S)-Fmoc-2-Amino-3-hydroxypropionic acid methyl ester
N-ALPHA-(9-FLUORENYLMETHOXYCARBONYL)-L-SERINE METHYL ESTER
(S)-Methyl 2-((((9H-fluoren-9-yl)Methoxy)carbonyl)aMino)-3-hydroxypropanoate
物理化学性质
制备方法
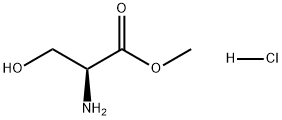
5680-80-8
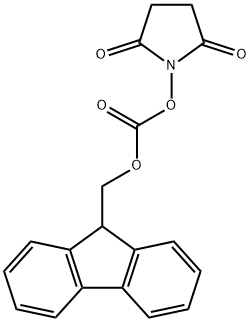
82911-69-1

82911-78-2
一般步骤:向含有L-丝氨酸甲酯盐酸盐(10.0g,64.3mmol)的1,4-二恶烷(15mL)和水(90mL)混合溶液中,加入碳酸氢钠(10.8g,129mmol)。将反应混合物于室温下搅拌15分钟。随后,向反应体系中缓慢加入2,5-二氧代吡咯烷-1-基9H-芴-9-基甲基碳酸酯(21.7g,64.3mmol)的1,4-二恶烷(60mL)溶液,继续在室温下搅拌14小时。反应完成后,向反应混合物中加入水,用乙酸乙酯萃取三次。合并有机层,依次用水和饱和盐水洗涤。有机层经无水硫酸镁干燥后过滤,减压浓缩。向残余物中加入乙醚和庚烷,过滤收集沉淀,得到fmoc-L-丝氨酸甲酯(22.3g,产率:定量)。产物经1H-NMR(400MHz,CDCl3)表征:δ 2.00-2.15(1H,m),3.81(3H,s),3.89-4.07(2H,m),4.20-4.28(1H,m),4.39-4.53(3H,m),5.63-5.74(1H,m),7.29-7.37(2H,m),7.38-7.46(2H,m),7.55-7.65(2H,m),7.74-7.82(2H,m)。
参考文献:
[1] Patent: US2015/175615, 2015, A1. Location in patent: Paragraph 0249 - 0251
[2] Patent: US2018/141950, 2018, A1. Location in patent: Paragraph 0100; 0101; 0102
[3] Journal of the American Chemical Society, 2007, vol. 129, # 7, p. 1884 - 1885
[4] Beilstein Journal of Organic Chemistry, 2017, vol. 13, p. 106 - 110
[5] Angewandte Chemie - International Edition, 2014, vol. 53, # 31, p. 8190 - 8194