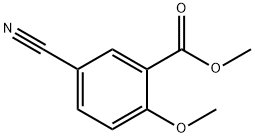
84923-71-7
 84923-71-7 结构式
84923-71-7 结构式
制备方法
40757-12-8

84923-71-7
实施例25B 5-氰基-2-甲氧基苯甲酸的合成:将实施例25A(5-氰基-2-甲氧基苯甲酸甲酯,6.1g,31.9mmol)和氢氧化锂一水合物(5.36g,128mmol)在四氢呋喃(100mL)和水(50mL)的混合溶剂中,于室温下搅拌反应3小时。反应完成后,用3N盐酸调节反应液pH至3,随后用异丙醇/二氯甲烷(1:3,v/v)混合溶剂萃取两次。合并有机相,用无水硫酸镁干燥,过滤后减压浓缩,得到5.6g(收率99%)目标产物5-氰基-2-甲氧基苯甲酸。产物经1H NMR(500MHz,氘代氯仿)表征,δppm:4.15(s,3H),7.17(d,J=8.85Hz,1H),7.86(dd,J=8.85,2.44Hz,1H),8.47(d,J=2.14Hz,1H)。
参考文献:
[1] Patent: US2008/287510, 2008, A1. Location in patent: Page/Page column 25
[2] Patent: WO2010/54024, 2010, A2. Location in patent: Page/Page column 74
[3] Patent: WO2008/130953, 2008, A2. Location in patent: Page/Page column 57-58
[4] Patent: US2008/242654, 2008, A1. Location in patent: Page/Page column 36
[5] Patent: EP1698618, 2006, A1. Location in patent: Page/Page column 78-79