85386-99-8
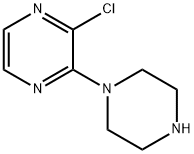 85386-99-8 结构式
85386-99-8 结构式
基本信息
2-氯-3-哌嗪-1-基吡嗪
2-氯-3-(1-哌嗪)吡嗪
2-氯-3-哌嗪-1-基-吡嗪
2-氯-3-(1-哌嗪基)吡嗪
2-氯-3-哌嗪吡嗪2-CHLORO-3-(1-PIPERAZINYL)PYRAZINE
2-氯-3-哌嗪吡嗪 2-Chloro-3-(1-piperazinyl)pyrazine
2-CHLORO-3-(PIPERAZINYL)PYRAZINE
2-CHLORO-3-(1-PIPERAZINYL)PYRAZINE
Pyrazine, 2-chloro-3-(1-piperazinyl)-
制备方法
![3'-氯-2,3,5,6-四氢-[1,2']联吡啶-4-羧酸叔丁酯](/CAS/GIF/313654-83-0.gif)
313654-83-0

85386-99-8
以4-(3-氯吡嗪-2-基)哌嗪-1-羧酸叔丁酯为原料合成2-氯-3-哌嗪吡嗪的一般步骤如下:将3'-氯-2,3,5,6-四氢-[1,2']联吡嗪基-4-羧酸叔丁酯(10g,33.4mmol,1当量)溶解于1,4-二恶烷(160mL)中,加入4M盐酸的1,4-二恶烷溶液(80mL,0.3mol,10当量)。在氮气保护下,室温搅拌反应混合物过夜。反应完成后,用二氯甲烷(600mL)稀释反应液,随后用50%氢氧化钠水溶液碱化。加入水(100mL)后,分离有机层和水层。水层用二氯甲烷(200mL)萃取两次。合并所有有机萃取液,用饱和氯化钠水溶液洗涤,无水硫酸镁干燥,过滤并浓缩,得到3'-氯-3,4,5,6-四氢-2H-[1,2']双吡嗪基产物,为粘稠油状物,静置后固化(6.39g,收率96%)。质谱(电喷雾离子化):m/z = 199.1, 201.1 [M + H]+。
参考文献:
[1] Patent: WO2009/29439, 2009, A1. Location in patent: Page/Page column 14
[2] Patent: WO2008/141020, 2008, A1. Location in patent: Page/Page column 64
[3] Patent: WO2009/48765, 2009, A1. Location in patent: Page/Page column 10
[4] Journal of Medicinal Chemistry, 2012, vol. 55, # 11, p. 5467 - 5482
[5] Patent: WO2017/21879, 2017, A1. Location in patent: Page/Page column 114-115
