861998-00-7
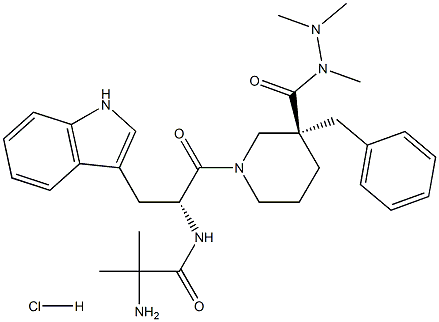 861998-00-7 结构式
861998-00-7 结构式
基本信息
阿拉莫林盐酸盐
饥饿素受体(GHRELIN RECEPTOR)激动剂(ANAMORELIN HYDROCHLORIDE)
AnaMorelin HCl
RC 1291 hydrochloride
ONO-7643 hydrochloride
AnaMorelin hydrochloride
Anamorelin (RC-1291) HCl
Anamorelin (RC-1291) hydrochloride
Anamorelin hydrochloride (RC-1291 hydrochloride
(3R)-1-(2-Methylalanyl-D-tryptophyl)-3-(phenylmethyl)-3-piperidinecarboxylic acid trimethylhydrazide monohydrochloride
RC-1291 HYDROCHLORIDE
ONO-7643 HYDROCHLORIDE
RC1291 HYDROCHLORIDE
ONO7643 HYDROCHLORIDE
RC 1291 HYDROCHLORIDE
ONO 7643 HYDROCHLORIDE
常见问题列表
阿拉莫林盐酸盐也被称为ONO-7643, RC-1291, 和 ST-1291,是一种由Novo Nordisk A/S开发,后由Helsinn Healthcare SA和Ono Pharmaceutical Co., Ltd.获得许可的口服活性药物。阿拉莫林盐酸盐用于治疗癌症恶病质和厌食症(cancer anorexia and cachexia syndrome, CACS),这是一种与癌症相关的副作用,表现为食欲减退、体重下降、肌肉消耗和生活质量下降。
阿拉莫林盐酸盐的合成方法如下:
2,2-二甲基肼(19.1)经甲酸乙酯处理后得到高收率的N-甲酰基二甲基肼19.2(图1)。然后用氢化铝锂还原醛获得产物 19.3,其为1,4-二噁烷溶液。中间体19.5的合成首先通过在常规条件下将尼泊金乙酯19.4保护为N-Boc衍生物开始,接着在低温条件下使用二异丙基酰胺锂形成烯醇负离子,并滴加溴化苄,得到了一个外消旋混合物19.5,经过三步反应的总收率为74%。接着,该酯经过皂化反应生成了酸19.6。外消旋混合物的手性分离通过向含19.6的乙酸乙酯(EtOAc)溶液中加入R-(+)-1-苯乙胺、水和三乙胺完成。通过过滤,从而以白色晶体形式分离出酸19.7。该产品的对映体过量值(ee)通过手性高效液相色谱(HPLC)测定为95.8%。关于三甲基肼19.3与酸19.7的偶联研究,原先采用PyBrop和DIPEA在室温下的THF中进行,反应时间需要延长至48小时,但所得的酰肼19.8的产率仅为65%。此外,由于所得固体的吸湿性,19.8的分离过程颇具挑战。尝试使用CDI和T3P进行酰胺键的形成均未成功,反应物未反应。EDCI和DMAP在二氯甲烷(DCM)中的使用也导致了分解产物的生成。最终,通过用草酰氯处理酸以活化成酸氯,加入催化量的DMF和三乙胺,再与19.3的5%溶液在THF或1,4-二氧六环中反应,成功合成了酰肼19.8。研究人员指出,试剂加入顺序对于成功生成酸氯至关重要,如果在加入草酰氯或DMF之前引入碱,则只能得到19.7及其分解产物。酸氯的预形成和随后与19.3溶液的反应对于后续产率(90%)获得千克级的19.8至关重要。最后,通过在乙酸乙酯(EtOAc)-盐酸气中溶解19.8去除Boc保护,形成了白色晶体固体19.9。
19.12的合成通过将氨基酸19.10和19.11在常规偶联条件下反应,随后用5%
NaOH于甲基叔丁基醚(MTBE)中进行皂化,得到了19.12,两步反应的总收率为86%。然后使用相同的偶联条件将酸19.12和胺19.9进行偶联,生成了酰胺19.13。对N-Boc保护基去除条件的筛选表明,使用甲磺酸在乙醇或甲醇中可以迅速去保护,且副产品形成最少。反应用温热的氢氧化钾水溶液淬灭后,慢慢冷却,得到游离碱的结晶,可以以超过99.5%的纯度分离出来。最后,通过将游离碱溶解在异丙醇醋酸酯中并加入化学当量的盐酸水溶液,形成了盐酸盐。混合物被轻轻加热后冷却至室温,得到了结晶态的Anamorelin单盐酸盐(19)。
Ki: 0.7 nM (ghrelin receptor)
EC50: 0.74 nM (ghrelin receptor)
In the FLIPR assay, Anamorelin (ANAM) shows significant agonist activity on the ghrelin receptor, with EC 50 value of 0.74 nM. No significant antagonist activity is observed with Anamorelin at concentrations of up to 1,000 nM. In the binding experiments, Anamorelin binds to the ghrelin receptor with a binding affinity constant (K i ) of 0.70 nM. In the competition assay with radiolabeled ibutamoren ( 35 S-MK-677; another ghrelin receptor agonist) Anamorelin (ANAM) is also found to bind with high affinity to the ghrelin receptor (IC 50 =0.69 nM). In rat pituitary cells incubated with Anamorelin, there is a dose-dependent stimulatory effect on GH release and the potency (EC 50 ) is 1.5 nM. Anamorelin is screened for activity against a set of over 100 receptors, ion channels, transporters, and enzymes. Anamorelin demonstrates binding to the tachykinin neurokinin 2 (NK 2 ) site (IC 50 =0.021 μM); however, a subsequent NK 2 functional assay demonstrates no functional activity.
In rats, Anamorelin (ANAM) at an oral dose of 3, 10, or 30 mg/kg once daily significantly increases both food intake and body weight from Day 2 to Day 7 of treatment compared with the vehicle control. The cumulative change in food intake and weight gain increases dose-dependently, and these changes are significant at all dose levels (P<0.05) compared to the control. Administration of Anamorelin at a single oral dose of 3, 10, or 30 mg/kg induces a dose-dependent increase in plasma GH levels and GH AUC 0-6h in rats.