864770-82-1
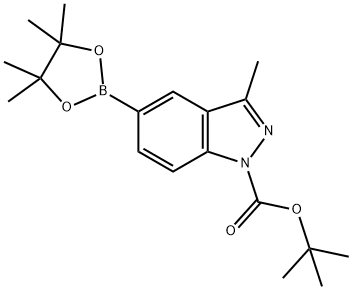 864770-82-1 结构式
864770-82-1 结构式
基本信息
1-N-叔丁氧羰基-3-甲基吲唑-5-硼酸频那醇酯
3-甲基-5-(4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)-1H-吲唑-1-羧酸叔丁酯
1-N-Boc-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-3-Methyl-indazole
(1-(TERT-BUTOXYCARBONYL)-3-METHYL-1H-INDAZOL-5-YL)BORONIC ACID PINACOL ESTER
3-Methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indazole-1-carboxylate
Tert-butyl 3-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)indazole-1-carboxylate
tert-butyl 3-Methyl-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-indazole-1-carboxylate
3-Methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indazole-1-carboxylic acid 1,1-dimethylethyl ester
1H-Indazole-1-carboxylic acid, 3-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-, 1,1-dimethylethyl ester
制备方法
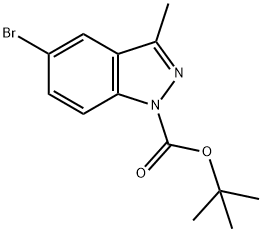
552331-49-4

73183-34-3

864770-82-1
以5-溴-3-甲基-1H-吲唑-1-甲酸叔丁酯(5.00g,16.1mmol)和联硼酸频那醇酯(4.49g,17.7mmol)为原料,加入乙酸钾(4.73g,48.3mmol)和Pd(dppf)Cl2(0.66g,0.81mmol)于无水DMF(60mL)中。将混合物置于带有橡胶衬里螺旋盖的小瓶中,通过三次真空/氮气循环进行脱气。随后,在氮气保护下,将悬浮液于100℃搅拌加热2小时。反应完成后,将混合物冷却至室温,与饱和碳酸氢钠水溶液(100mL)混合,并用乙酸乙酯(2×150mL)萃取。合并有机相,经无水硫酸钠干燥后,浓缩得到粗产物。粗产物通过硅胶柱色谱纯化,采用乙酸乙酯/己烷(0%至15%)梯度洗脱,得到目标产物3-甲基-5-(4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)-1H-吲唑-1-羧酸叔丁酯,为油状物(5.55g,收率96%)。LCMS(+APCI)m/z: 499(M+H)。
参考文献:
[1] Patent: US2008/153813, 2008, A1. Location in patent: Page/Page column 24
[2] Patent: WO2005/85227, 2005, A1. Location in patent: Page/Page column 37; 76
[3] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 2, p. 673 - 678
[4] Patent: US2008/153813, 2008, A1. Location in patent: Page/Page column 22-23
[5] Patent: US2008/153813, 2008, A1. Location in patent: Page/Page column 23
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW0286477082101 | 3-甲基-5-(4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)-1H-吲唑-1-羧酸叔丁酯 | 864770-82-1 | 100MG | 106元 |
| 2025/05/22 | XW0286477082103 | 3-甲基-5-(4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)-1H-吲唑-1-羧酸叔丁酯 | 864770-82-1 | 1G | 606元 |
| 2025/05/22 | XW0286477082102 | 3-甲基-5-(4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)-1H-吲唑-1-羧酸叔丁酯 | 864770-82-1 | 250MG | 264元 |
