869478-09-1
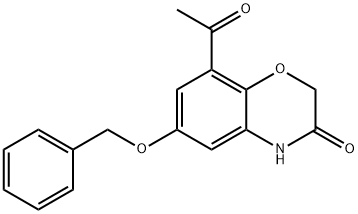 869478-09-1 结构式
869478-09-1 结构式
基本信息
8-乙酰基-6-苄氧基-4H-苯并[1,4]恶嗪-3-酮
3-羰基-8-乙酰基-6-苄氧基-4氢-苯并[1,4]恶嗪
6-(苄氧基)-8-乙酰基-2H-苯并[1,4]恶嗪-3(4H)-酮
8-乙酰基-6-(苄氧基)-2H-苯并[B][1,4]噁嗪-3(4H)-酮
8-acetyl-6-benzyloxy-4H-benzo[1,4]oxazin-3-one
8-acetyl-6-phenylmethoxy-4H-1,4-benzoxazin-3-one
8-acetyl-6-(phenylmethoxy)-2H-1,4-benzoxazin-3(4H)-one
8-acetyl-6-(benzyloxy)-2H-benzo[b][1,4]oxazin-3(4H)-one
2H-1,4-Benzoxazin-3(4H)-one, 8-acetyl-6-(phenylMethoxy)-
8- Acetyl-6 - (benzoxy) -2H- Benzo [b] [1,4] oxazine -3 (4H) -ONE
物理化学性质
制备方法
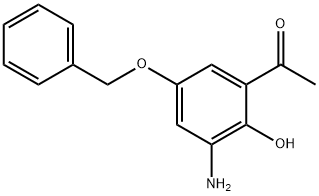
861841-90-9

79-04-9
![6-(苄氧基)-8-乙酰基-2H-苯并[1,4]恶嗪-3(4H)-酮](/CAS/GIF/869478-09-1.gif)
869478-09-1
以1-(3-氨基-5-(苄氧基)-2-羟基苯基)乙酮和氯乙酰氯为原料,合成8-乙酰基-6-(苄氧基)-2H-苯并[b][1,4]噁嗪-3(4H)-酮的一般步骤如下:在冰浴冷却条件下,将21.0 mL(258 mmol)氯乙酰氯缓慢滴加到含有60.0 g(233 mmol)1-(3-氨基-5-苄氧基-2-羟基苯基)乙酮和70.0 g(506 mmol)碳酸钾的混合物中。滴加完毕后,将反应混合物在室温下搅拌过夜,随后加热至回流温度继续搅拌6小时。反应完成后,趁热过滤反应混合物,并将滤液浓缩至约400 mL。向浓缩液中加入冰水,析出沉淀。通过抽滤收集沉淀,干燥后,使用短硅胶柱(洗脱剂比例为二氯甲烷:甲醇=99:1)进行色谱纯化。合并含有目标产物的馏分,蒸发溶剂后,将残余物悬浮于异丙醇/二异丙醚混合溶剂中,抽滤并用二异丙醚洗涤,得到纯品。产量:34.6 g(收率50%);质谱数据:[M + H]+ = 298。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 4, p. 1410 - 1414
[2] Patent: US2005/267106, 2005, A1. Location in patent: Page/Page column 7
[3] Patent: WO2005/111005, 2005, A1. Location in patent: Page/Page column 16
[4] Patent: US2007/249595, 2007, A1. Location in patent: Page/Page column 11
[5] Patent: US2007/88160, 2007, A1. Location in patent: Page/Page column 11
