870843-42-8
中文名称
(E)-1-[(1S)-1-(4-氟苯基)乙基]-3-[3-甲氧基-4-(4-甲基-1H-咪唑-1-YL)亚苄基]哌啶-2-酮
英文名称
(E)-1-[(1S)-1-(4-Fluorophenyl)ethyl]-3-[3-methoxy-4-(4-methyl-1H-imidazol-1-yl)benzylidene]piperidin-2-one
CAS
870843-42-8
分子式
C25H26FN3O2
分子量
419.49
MOL 文件
870843-42-8.mol
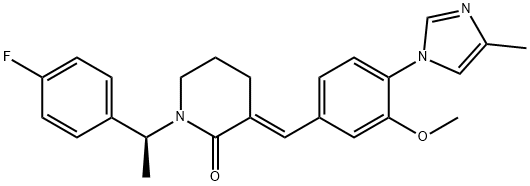 870843-42-8 结构式
870843-42-8 结构式
基本信息
中文别名
(E)-1-[(1S)-1-(4-氟苯基)乙基]-3-[3-甲氧基-4-(4-甲基-1H-咪唑-1-基)亚苄基]哌啶-2-酮(E)-1-[(1S)-1-(4-氟苯基)乙基]-3-[3-甲氧基-4-(4-甲基-1H-咪唑-1-YL)亚苄基]哌啶-2-酮
英文别名
E 2012CS-510
E-2012
E2012
E2012
E 2012
E-2012
1-[(1S)-1-(4-fluorophenyl)ethyl]-3-[[3-Methoxy-4-(4-Methyl-1H-iMidazol-1-yl)phenyl]Methylene]-
(E)-1-[(1S)-1-(4-Fluorophenyl)ethyl]-3-[3-methoxy-4-(4-methyl-1H-imidazol-1-yl)benzylidene]pip
(E)-1-[(1S)-1-(4-Fluorophenyl)ethyl]-3-[3-methoxy-4-(4-methyl-1H-imidazol-1-yl)benzylidene]piperi
(S,E)-1-(1-(4-fluorophenyl)ethyl)-3-(3-methoxy-4-(4-methyl-1H-imidazol-1-yl)benzylidene)piperidin-2-one
(E)-1-[(1S)-1-(4-Fluorophenyl)ethyl]-3-[3-methoxy-4-(4-methyl-1H-imidazol-1-yl)benzylidene]piperidin-2-one
(E)-1-[(IS)-1-(4-fluorophenyl)ethyl]-3-[3-methoxy-4-(4-methyl-1H-imidazol-1 -y1) benzylidene]piperidin-2-one
所属类别
生物化工:激动剂抑制剂物理化学性质
沸点649.2±55.0 °C(Predicted)
密度1.19±0.1 g/cm3(Predicted)
储存条件-20°C储存
溶解度DMF: 10mg/mL; DMSO: 10mg/mL; DMSO:PBS (pH 7.2) (1:3): 0.25mg/mL; Ethanol: 10mg/mL
酸度系数(pKa)5.67±0.61(Predicted)
形态粉末
颜色Light yellow to yellow
(E)-1-[(1S)-1-(4-氟苯基)乙基]-3-[3-甲氧基-4-(4-甲基-1H-咪唑-1-YL)亚苄基]哌啶-2-酮价格(试剂级)
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-10016 | (E)-1-[(1S)-1-(4-氟苯基)乙基]-3-[3-甲氧基-4-(4-甲基-1H-咪唑-1-YL)亚苄基]哌啶-2-酮 E 2012 | 870843-42-8 | 1 mg | 310元 |
| 2025/12/22 | HY-10016 | (E)-1-[(1S)-1-(4-氟苯基)乙基]-3-[3-甲氧基-4-(4-甲基-1H-咪唑-1-YL)亚苄基]哌啶-2-酮 E 2012 | 870843-42-8 | 10mM * 1mLin DMSO | 904元 |
| 2025/12/22 | HY-10016 | (E)-1-[(1S)-1-(4-氟苯基)乙基]-3-[3-甲氧基-4-(4-甲基-1H-咪唑-1-YL)亚苄基]哌啶-2-酮 E 2012 | 870843-42-8 | 5mg | 980元 |
常见问题列表
生物活性
E 2012 是有效的 γ 分泌酶调节剂,不会影响 Notch 加工。E 2012 在胆固醇生物合成的最后一步抑制 3β-羟基固醇 Δ24-还原酶 (DHCR24)。E 2012 旨在通过减少淀粉样蛋白 β-42 减少阿兹海默氏病,并在大鼠多次重复给药后诱发白内障。体外研究
E2012 has concentration-dependent inhibitory effects on cholesterol biosynthesis in primary culture of rat hepatocytes and HepG2 cells with IC 50 s of 11.0, and 15.1 nM, respectively.
体内研究
In vivo lenticular concentration of E 2012 after 13-week repeated dose with cataract was well above those where inhibition is observed in vitro. E 2012 induces cataract in the rat by inhibiting DHCR24 at the final step of cholesterol synthesis with associated elevation in desmosterol within the lens, preceded by desmosterol changes that would serve as a predictive safety biomarker for lenticular opacity.
