875781-41-2
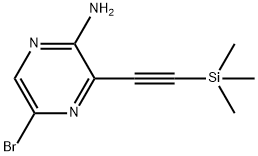 875781-41-2 结构式
875781-41-2 结构式
基本信息
3-[(三甲基硅基)炔基]-5-溴吡嗪-2-胺
3-[(三甲基硅基)乙炔基]-5-溴吡嗪-2-胺
5-Bromo-3-((trimethylsilyl)
5-Bromo-3-trimethylsilanylethynylpyrazin-2-ylamine
5-bromo-3-((trimethylsilyl)ethynyl)pyrazin-2-amine
Pyrazinamine, 5-bromo-3-[(trimethylsilyl)ethynyl]-
5-bromo-3-[2-(trimethylsilyl)ethynyl]-2-Pyrazinamine
5-broMo-3-(2-(triMethylsilyl)ethynyl)pyrazin-2-aMine
物理化学性质
制备方法
24241-18-7
1066-54-2
![3-[(三甲基硅基)乙炔基]-5-吡嗪-2-胺](/CAS2/GIF/875781-41-2.gif)
875781-41-2
向3,5-二溴吡嗪-2-胺(2.00 g,7.91 mmol)的无水THF(24 mL)溶液中依次加入三乙胺(3.3 mL,24 mmol)、碘化亚铜(151 mg,0.79 mmol)和Pd(PPh3)2Cl2(催化量)。在氮气保护下,将反应混合物冷却至-5℃,然后缓慢滴加三甲基硅乙炔(1.07 mL,7.50 mmol)。滴加完毕后,将反应体系缓慢升温至0℃,并在此温度下搅拌1.5小时。反应完成后,将混合物在减压下浓缩以除去溶剂,所得残余物通过硅胶柱色谱法(洗脱剂:石油醚/乙酸乙酯,体积比5:1)纯化,得到目标产物3-[(三甲基硅基)炔基]-5-溴吡嗪-2-胺,为黑色油状物(42 mg,收率94%)。质谱(ESI,正离子模式)m/z:272.00 [M + H]+;1H NMR(400 MHz,CDCl3)δ(ppm):8.04(s,1H),5.14(s,2H),0.30(s,9H)。
参考文献:
[1] Patent: WO2018/108125, 2018, A1. Location in patent: Paragraph 00586
[2] Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 3, p. 693 - 698
[3] Bioorganic and Medicinal Chemistry Letters, 2015, vol. 25, # 20, p. 4399 - 4404
[4] Patent: CN106432246, 2017, A. Location in patent: Paragraph 0540; 0541; 0542
[5] Patent: WO2010/54398, 2010, A1. Location in patent: Page/Page column 150-151