884512-77-0
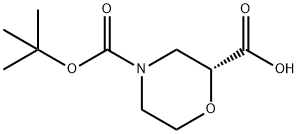 884512-77-0 结构式
884512-77-0 结构式
基本信息
(R)-N-BOC-2-吗啉甲酸
(R)-4-BOC-吗啉-2-羧酸
(2(R)-N-BOC-吗啉2-甲酸
(2R)-2,4-吗啉二羧酸 4-叔丁酯
(R)-4-(叔丁氧羰基)吗啉-2-羧酸
(R)-4-(叔丁氧基羰基)吗啉-2-羧酸
(R)-4-Boc-morpholine-2-carboxylic acid
(R)/(S)-N-Boc-2-morpholinecarboxylic acid
(R)-N-BOC-2,4-MORPHOLINEDICARBOXYLIC ACID
(2R)-4-BOC-2,4-MORPHOLINEDICARBOXYLIC ACID
(2R)-4-(tert-butoxycarbonyl)morpholin-2-carboxylic acid
(R)-4-(tert-butoxycarbonyl)morpholine-2-carboxylic acid
(2R)-4-(tert-butoxycarbonyl)morpholine-2-carboxylic acid
(2R)-4-(1,1-Dimethylethyl) Ester 2,4-Morpholinedicarboxylic Acid
(2R)-2,4-Morpholinedicarboxylic acid 4-(1,1-dimethylethyl) ester
物理化学性质
制备方法
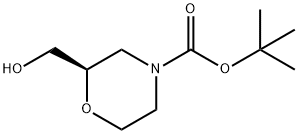
135065-71-3

884512-77-0
以(R)-N-Boc-2-羟甲基吗啉为原料合成(2R)-2,4-吗啉二羧酸4-叔丁酯的一般步骤:在0℃下,将饱和NaHCO3水溶液(15mL)加入至(R)-2-(羟甲基)-吗啉-4-羧酸叔丁酯(1.09g,5.0mmol)的丙酮(50mL)溶液中,持续搅拌。随后加入固体NaBr(0.1g,1mmol)和TEMPO(0.015g,0.1mmol)。在0℃下,于20分钟内缓慢滴加三氯异氰尿酸(2.32g,10.0mmol)。滴加完毕后,将反应混合物缓慢升温至室温并搅拌过夜。反应完成后,加入2-丙醇(3mL),室温下继续搅拌30分钟。反应液经硅藻土垫过滤,滤液在减压下浓缩。浓缩物用饱和Na2CO3水溶液(15mL)处理,水相用EtOAc(5mL)洗涤后,用6N HCl酸化,再用EtOAc(5×10mL)萃取。合并有机层,用Na2SO4干燥,减压除去溶剂,得到(R)-4-(叔丁氧基羰基)吗啉-2-甲酸(1.07g,收率92%)为白色固体。产物经1H NMR(400MHz,CDCl3)确认:δ 4.20(br,1H),4.12(d,1H),4.02(d,1H),3.84(m,1H),3.62(m,1H),3.04(m,2H),1.44(s,9H);质谱(MS)m/z 232(M+H+)。
参考文献:
[1] Patent: WO2008/124575, 2008, A1. Location in patent: Page/Page column 112; 114
[2] Patent: US2010/184805, 2010, A1. Location in patent: Page/Page column 30-31
[3] Patent: WO2007/70201, 2007, A1. Location in patent: Page/Page column 176-177
[4] Patent: US2010/317697, 2010, A1. Location in patent: Page/Page column 51
[5] Patent: WO2008/124582, 2008, A1. Location in patent: Page/Page column 96-98
