885681-96-9
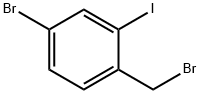 885681-96-9 结构式
885681-96-9 结构式
基本信息
2-碘-4-溴-苄溴
4-溴-2-碘苄基溴
4-溴-1-(溴甲基)-2-碘苯
4-Bromo-2-iodobenzylbromide97%
4-Bromo-2-iodobenzyl bromide 97%
4-Bromo-1-(bromomethyl)-2-iodobenzene
Benzene, 4-bromo-1-(bromomethyl)-2-iodo-
4-Bromo-1-(bromomethyl)-2-iodobenzene, alpha,4-Dibromo-2-iodotoluene
物理化学性质
制备方法
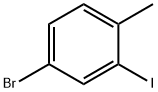
260558-15-4

885681-96-9
以4-溴-2-碘-1-甲基苯为原料合成4-溴-1-(溴甲基)-2-碘苯(15b)的一般步骤:在氮气保护下,将4-溴-2-碘甲苯(3.94 g,13.3 mmol)溶于1,2-二氯乙烷(19 mL)中,置于50 mL双颈圆底烧瓶中,室温搅拌。随后依次加入过氧化苯甲酰(164 mg,0.663 mmol)和N-溴代琥珀酰亚胺(NBS,2.63 g,14.6 mmol),将反应混合物加热回流5小时。反应完成后,冷却至室温,过滤收集沉淀,并用己烷洗涤。滤液经无水硫酸镁(MgSO4)干燥后,过滤并减压浓缩,得到橙色固体。该固体用冷甲醇冲洗,得到灰白色固体产物(3.27 g,8.75 mmol,收率66%),无需进一步纯化即可用于后续反应。产物结构经1H-NMR(400 MHz,CDCl3)确认:δ 8.01(d,1H,J = 2.0 Hz),7.47(dd,1H,J = 8.3, 2.0 Hz),7.33(d,1H,J = 8.3 Hz),4.54(s,2H);高分辨质谱(HRMS,EI)m/z计算值C7H5Br2I [M+]:377.7762,实测值:377.7759。
参考文献:
[1] Patent: TW2016/9616, 2016, A. Location in patent: Paragraph 0162
[2] Journal of Organic Chemistry, 2011, vol. 76, # 7, p. 2227 - 2239
[3] Synthesis, 2011, # 15, p. 2387 - 2391
[4] Patent: WO2011/127383, 2011, A2. Location in patent: Page/Page column 74
[5] Angewandte Chemie - International Edition, 2014, vol. 53, # 7, p. 1841 - 1844
