887757-48-4
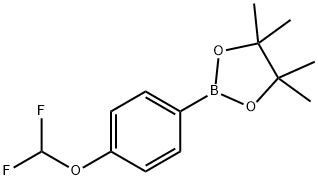 887757-48-4 结构式
887757-48-4 结构式
基本信息
4-(二氟甲氧基)苯硼酸频哪醇酯
4-(二氟甲氧基)苯基硼酸频哪醇酯
2-(4-(二氟甲氧基)苯基)-硼酸频哪醇酯
4-Difluoromethoxyphenylboronic acid pinacol ester
2-[4-(difluoromethoxy)phenyl]-4,4,5,5-tetramethyl-1,3,2-diox...
2-(4-(difluoromethoxy)phenyl)-4,4,5-trimethyl-1,3,2-dioxaborolane
2-(4-(DifluoroMethoxy)phenyl)-4,4,5,5-tetraMethyl-1,3,2-dioxaborolane
1,3,2-Dioxaborolane, 2-[4-(difluoromethoxy)phenyl]-4,4,5,5-tetramethyl-
物理化学性质
制备方法
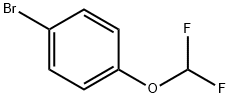
5905-69-1

73183-34-3

887757-48-4
A. 合成2-(4-(二氟甲氧基)苯基)-4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷的一般步骤: 在氮气保护下,将1-溴-4-(二氟甲氧基)苯(1g,4.48mmol)、联硼酸频那醇酯(1.2g,4.71mmol)、乙酸钾(1.3g,13.4mmol)和PdCl2(dppf)-CH2Cl2加合物(0.18g,0.22mmol)溶于DMF(15mL)中,于90℃搅拌反应1小时。反应完成后,用二氯甲烷稀释反应混合物,依次用水、饱和NaHCO3溶液和盐水洗涤,有机相用无水MgSO4干燥,浓缩。粗产物通过ISCO快速色谱法(硅胶柱,洗脱剂为100:0至50:50的己烷/乙酸乙酯梯度)纯化,得到目标产物2-(4-(二氟甲氧基)苯基)-4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷(1.12g,3.32mmol,产率74.0%),为棕色油状物。
参考文献:
[1] Organic Process Research and Development, 2018, vol. 22, # 4, p. 557 - 561
[2] Patent: WO2010/104830, 2010, A1. Location in patent: Page/Page column 98-99
[3] Patent: US2012/329802, 2012, A1. Location in patent: Page/Page column 33
[4] Patent: US2007/60623, 2007, A1. Location in patent: Page/Page column 49
[5] Patent: WO2009/26276, 2009, A1. Location in patent: Page/Page column 49-50
