893567-09-4
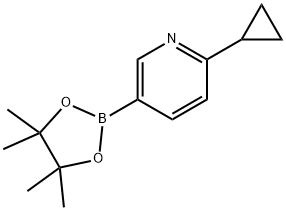 893567-09-4 结构式
893567-09-4 结构式
基本信息
6-环丙基-3-吡啶硼酸频哪醇酯
6-环丙基吡啶-3-硼酸频那醇酯 1G
2-环丙基-5-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)吡啶
6-Cyclopropylpyridine-3-boronic acid pinacol ester
2-Cyclopropyl-5-pyridinyl boronic acid pinacol ester
6-CYCLOPROPYL-3-PYRIDINYL BORONIC ACID PINACOL ESTER
2-Cyclopropyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)
2-cyclopropyl-5-(tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine
Pyridine, 2-cyclopropyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
6-Cyclopropylpyridine-3-boronic acid pinacol ester 6-(Cyclopropyl)pyridine-3-boronic acid pinacol ester
物理化学性质
制备方法

73183-34-3
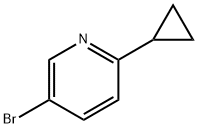
579475-29-9

893567-09-4
以5-溴-2-环丙基吡啶(0.21g,1.1mmol)、双(频哪醇合)二硼(0.31g,1.2mmol)和乙酸钾(0.32g,3.2mmol)为原料,在二恶烷(10mL)中制备悬浮液。将悬浮液用氩气脱气10分钟后,加入PdCl2·dppf(0.026g)。将反应混合物加热至80℃,保持15小时。反应完成后,冷却至室温,通过硅藻土塞过滤。浓缩滤液,得到黑色油状物。将该油状物溶于乙醚中,用1.0M氢氧化钠溶液萃取四次。合并的黄色水相冷却至10℃,用2.5M盐酸调节pH至6.5,随后用乙醚重复萃取。合并有机相,用无水硫酸镁干燥,过滤并浓缩,得到0.27g(103%)的6-环丙基吡啶-3-硼酸频哪醇酯,为黄色油状物,其缓慢固化。1H-NMR分析显示产物纯度约为60-65%,主要杂质为频哪醇硼烷。粗产物可直接用于后续反应,无需进一步纯化。GC-MS m/z 245.2(M+),244.2(M-I);1H-NMR(CDCl3)δ 8.78(br s,1H),7.93(dd,1H),7.10(d,1H),2.10(m,1H),1.34(s,12H),1.10-1.00(m,4H)ppm。
参考文献:
[1] Patent: WO2006/65215, 2006, A1. Location in patent: Page/Page column 29
[2] Patent: WO2015/52264, 2015, A1. Location in patent: Paragraph 0556; 0557
[3] Patent: US2018/296543, 2018, A1. Location in patent: Page/Page column 0731; 0732
