89901-03-1
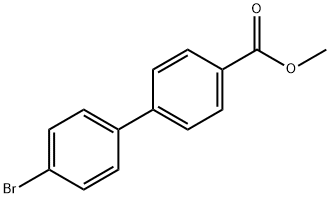 89901-03-1 结构式
89901-03-1 结构式
基本信息
4'-溴联苯-4-甲酸甲酯
4'-溴联苯-4-甲酸甲酯
4'-溴代联苯-4-羧酸甲酯
4'-溴代联苯-4-羧酸甲酯
4‘-溴(1,1-联苯)-4-甲酸甲脂
4'-溴[1,1'-联苯]-4-甲酸甲酯
4'-溴-[1,1'-联苯] -4-甲酸甲酯
Methyl 4-(4-bromophenyl)benzoate
Methyl 4'-bromobiphenyl-4-carboxylate
Methyl 4'-bromobiphenyl-4-carboxylate, 95%
METHYL 4'-BROMO[1,1'-BIPHENYL]-4-CARBOXYLATE
methyl 4'-bromo-[1,1'-biphenyl]-4-carboxylate
4′-Bromobiphenyl-4-carboxylic acid methyl ester
Methyl 4'-bromo-[1,1'-biphenyl]-4-carboxylate 95%
[1,1'-Biphenyl]-4-carboxylicacid, 4'-bromo-, methyl ester
Methyl 4-(4-bromophenyl)benzoate, 4'-Bromo-4-(methoxycarbonyl)biphenyl
物理化学性质
安全数据
Aquatic Chronic 1
Eye Dam. 1
制备方法
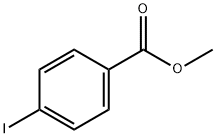
619-44-3
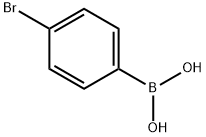
5467-74-3
![4'-溴[1,1'-联苯]-4-甲酸甲酯](/CAS/GIF/89901-03-1.gif)
89901-03-1
以4-碘苯甲酸甲酯(9.38 g,35.8 mmol)和4-溴苯硼酸(7.18 g,35.8 mmol)为原料,加入Pd(PPh3)4(2.07 g,1.79 mmol)于甲苯(180 mL)和乙醇(100 mL)的混合溶剂中,形成澄清溶液。向该溶液中加入4.0 M碳酸钠水溶液(30 mL)。将反应混合物在80℃下回流4小时。反应完成后,将混合物冷却至室温,并用乙酸乙酯(300 mL)稀释。依次用水(2×300 mL)、饱和NaHCO3水溶液(2×300 mL)和饱和NaCl水溶液洗涤有机层,然后用MgSO4干燥。浓缩有机相后,通过柱色谱法(洗脱剂:7%乙酸乙酯-正庚烷)纯化残余物,得到白色固体产物4'-溴[1,1'-联苯]-4-甲酸甲酯(7.8 g,收率78%)。1H NMR (CDCl3) δ: 8.10 (d, 2H, J = 9.0 Hz), 7.62 (d, 2H, J = 9.0 Hz), 7.59 (d, 2H, J = 9.3 Hz), 7.48 (d, 2H, J = 9.3 Hz), 3.95 (s, 3H)。
参考文献:
[1] Patent: WO2004/99170, 2004, A2. Location in patent: Page 57
[2] Patent: WO2006/55625, 2006, A2. Location in patent: Page/Page column 94-95
[3] Patent: WO2006/55725, 2006, A2. Location in patent: Page/Page column 179
[4] Patent: WO2004/99171, 2004, A2. Location in patent: Page 116-117

