90923-75-4
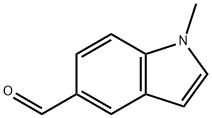 90923-75-4 结构式
90923-75-4 结构式
基本信息
5-Formyl-1-methyl-1H-indole
1-Methylindole-5-carboxaldehyde
1-METHYL-1H-INDOLE-5-CARBALDEHYDE
1-METHYL-1H-INDOLE-5-CARBOXALDEHYDE
1H-Indole-5-carboxaldehyde, 1-methyl-
1-Methyl-1H-indole-5-carboxaldehyde97%
1-Methyl-1H-indole-5-carboxaldehyde 97%
1H-Indole-5-carboxaldehyde, 1-methyl- (9CI)
1-methyl-1H-indole-5-carbaldehyde(SALTDATA: FREE)
物理化学性质
安全数据
制备方法
1196-69-6

74-88-4

90923-75-4
向5-吲哚甲醛(1.0g,6.9mmol)的N,N-二甲基甲酰胺(DMF,15mL)溶液中分批加入氢化钠(303mg,60%油分散体,7.59mmol)。将反应混合物在室温下搅拌30分钟。随后,向反应体系中加入碘甲烷(1.96g,13.8mmol),并将混合物继续搅拌过夜。反应完成后,加入乙酸乙酯(200mL)稀释反应混合物,依次用水(3×20mL)和饱和食盐水(25mL)洗涤有机相。有机相用无水硫酸镁干燥,过滤后浓缩,得到1-甲基-1H-吲哚-5-甲醛,为橙色油状物(1.0g,收率91%)。产物经1H NMR(200MHz,CDCl3)表征:δ 10.05(s,1H),8.09(s,1H),7.90-7.80(m,1H),7.35-7.15(m,2H),6.85-6.80(m,1H),3.95(s,3H)。
参考文献:
[1] Patent: WO2004/52890, 2004, A1. Location in patent: Page 91-92
[2] Bioorganic and Medicinal Chemistry, 2014, vol. 22, # 7, p. 2060 - 2079
[3] European Journal of Medicinal Chemistry, 2018, vol. 158, p. 167 - 183
[4] Patent: US2005/209274, 2005, A1. Location in patent: Page/Page column 27
[5] Journal of Organometallic Chemistry, 2011, vol. 696, # 5, p. 1072 - 1083
