91103-37-6
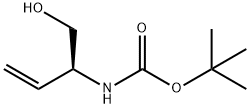 91103-37-6 结构式
91103-37-6 结构式
基本信息
(S)-叔丁基- 1-羟基丁-3-烯-2-基碳酸酯
(S)-(1-羟基丁-3-烯-2-基)氨基甲酸叔丁酯
(S)-tert-butyl 1-hydroxybut-3-en-2-ylcarbamate
tert-butyl (S)-(1-hydroxybut-3-en-2-yl)carbamate
CarbaMic acid, [(1S)-1-(hydroxyMethyl)-2-propenyl]-, 1,1-diMethylethylester
Carbamic acid, N-[(1S)-1-(hydroxymethyl)-2-propen-1-yl]-, 1,1-dimethylethyl ester
物理化学性质
制备方法
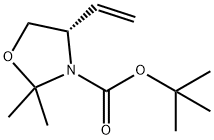
133625-87-3

91103-37-6
以 (S)-2,2-二甲基-4-乙烯基恶唑烷-3-羧酸叔丁酯为原料合成 (S)-(1-羟基丁-3-烯-2-基)氨基甲酸叔丁酯的一般步骤:在0℃下,向 (4S)-2,2-二甲基-4-乙烯基-1,3-恶唑烷-3-羧酸叔丁酯(1.90 g,8.36 mmol)的甲醇(83 mL)溶液中加入对甲苯磺酸一水合物(0.80 g,4.2 mmol)。将反应混合物缓慢升温至室温并搅拌过夜。反应完成后,用饱和NaHCO3溶液稀释反应混合物,然后浓缩。用乙酸乙酯萃取混合物,有机层依次用饱和NaHCO3水溶液洗涤两次,再用盐水洗涤。有机层用Na2SO4干燥,过滤并浓缩,得到目标产物 (S)-(1-羟基丁-3-烯-2-基)氨基甲酸叔丁酯,为无色油状物(1.187 g,收率76%)。产物的结构通过1H NMR(400 MHz,CDCl3)确认:δ 5.81(1H,m),5.25(2H,m),4.90(1H,m),4.25(1H,br s),3.67(2H,m),1.45(9H,s)ppm。
参考文献:
[1] Patent: US2014/121198, 2014, A1. Location in patent: Paragraph 0645; 0647
[2] Patent: WO2015/26818, 2015, A1. Location in patent: Page/Page column 44
[3] Patent: WO2015/157257, 2015, A1. Location in patent: Page/Page column 37; 38
[4] Patent: US2015/361094, 2015, A1. Location in patent: Paragraph 0354
[5] Tetrahedron Asymmetry, 2001, vol. 12, # 6, p. 817 - 819
