914988-10-6
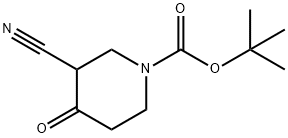 914988-10-6 结构式
914988-10-6 结构式
基本信息
N-BOC-3-氰基-4-哌啶酮
1-BOC-4-氧代哌啶-3-甲腈
3-氰基-4-氧代哌啶-1-羧酸叔丁酯
3-氰基-4-氧代-1-哌啶羧酸叔丁酯
3-氰基-4-氧代-哌啶-1-羧基酸叔丁酯
1-Boc-4-oxopiperidine-3-carbonitrile
tert-butyl 4-cyano-3-oxopiperidine-1-carboxylate
TERT-BUTYL 3-CYANO-4-OXOPIPERIDINE-1-CARBOXYLATE
Tert-butyl 3-cyano-4-oxo-1-piperidinecarboxylate
3-cyano-4-oxo-1-piperidinecarboxylic acid tert-butyl ester
3-CYANO-4-OXO-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
3-Cyano-4-Oxo-1-Piperidinecarboxylic Acid 1,1-Dimethylethyl Ester
1-Piperidinecarboxylic acid,3-cyano-4-oxo-,1,1-dimethylethyl ester
物理化学性质
制备方法
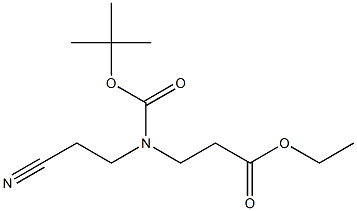
266353-22-4

914988-10-6
以3-((叔丁氧基羰基)(2-氰基乙基)氨基)丙酸乙酯为原料合成N-Boc-氰基-哌啶-4-酮的一般步骤如下:将3-((叔丁氧基羰基)(2-氰基乙基)氨基)丙酸乙酯(17.3g,64.0mmol)溶解于200mL甲苯中,加入60%的氢化钠(3.84g,96.0mmol)。将反应混合物加热至回流并维持3小时。反应完成后,冷却至室温,用200mL水稀释,随后用1N盐酸溶液调节pH至3。水相用乙酸乙酯(200mL×3)萃取。合并有机相,用无水硫酸钠干燥,过滤后减压浓缩。残余物通过硅胶柱色谱纯化,以乙酸乙酯/石油醚为洗脱剂,得到7.8g(产率51.6%)的3-氰基-4-氧代哌啶-1-羧酸叔丁酯,为白色固体。1H NMR(400MHz,DMSO-d6)δ10.87(s,1H),3.89(s,2H),3.46(t,J=5.8Hz,2H),2.28(t,J=5.8Hz,2H)。
参考文献:
[1] Journal of Medicinal Chemistry, 2017, vol. 60, # 22, p. 9162 - 9183
[2] Patent: US2015/5277, 2015, A1. Location in patent: Paragraph 0208; 0209
[3] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 17, p. 5195 - 5198
[4] Molecules, 2011, vol. 16, # 3, p. 2626 - 2635
[5] Patent: WO2016/86200, 2016, A1. Location in patent: Page/Page column 139; 140; 519; 520

