916325-83-2
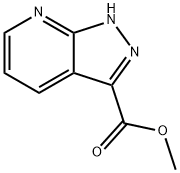 916325-83-2 结构式
916325-83-2 结构式
基本信息
1H-吡唑并[3,4-B]吡啶-3-羧酸甲酯
2H-吡唑并[3,4-B]吡啶-3-羧酸甲酯
1H-吡唑并[3,4-B]吡啶-3-甲酸甲酯
甲基 1H-吡唑并[3,4-B]吡啶-3-甲酸基酯
1H-pyrazolo[3,4-b]pyridine-3-carboxylate
Methyl 1H-pyrazolo[3,4-b]pyridine-3-carboxylate
Methyl 2H-pyrazolo[3,4-b]pyridine-3-carboxylate
pyrazolo[3,4-b]pyridine-3-carboxylic acid methyl ester
2H-pyrazolo[3,4-b]pyridine-3-carboxylic acid methyl ester
1H-Pyrazolo[3,4-b]pyridine-3-carboxylic acid, Methyl ester
物理化学性质
制备方法

67-56-1
![1H-吡唑基[3,4-B]吡啶-3-羧酸](/CAS/GIF/116855-08-4.gif)
116855-08-4
![1H-吡唑并[3,4-B]吡啶-3-羧酸甲酯](/CAS/GIF/916325-83-2.gif)
916325-83-2
步骤5:向1H-吡唑并[3,4-b]吡啶-3-羧酸(V)(0.39 g,2.4 mmol)的无水甲醇(10 mL)溶液中缓慢加入浓硫酸(4滴),并在氮气保护下回流反应6小时。反应完成后,将反应液冷却至室温,随后在减压下蒸发除去溶剂。将残余物在二氯甲烷和饱和碳酸氢钠溶液之间进行分配。分离有机层,用无水硫酸镁干燥,过滤并减压浓缩。粗产物通过快速硅胶柱色谱法(洗脱梯度:100%二氯甲烷→100:3二氯甲烷:甲醇)纯化,得到1H-吡唑并[3,4-b]吡啶-3-羧酸甲酯(VI),为白色固体(382 mg,2.16 mmol,收率90%)。1H NMR(CDCl3)δ ppm:4.08(s,3H),7.38(m,1H),8.63(dd,J = 8.10, 1.51 Hz,1H),8.72(dd,J = 4.62, 1.41 Hz,1H);ESIMS m/z:178.2([M + H]+)。
参考文献:
[1] Patent: WO2011/84486, 2011, A1. Location in patent: Page/Page column 106; 112
[2] Patent: US2013/296302, 2013, A1. Location in patent: Paragraph 0497
[3] Patent: US2016/68550, 2016, A1. Location in patent: Paragraph 1059
[4] Patent: WO2016/40193, 2016, A1. Location in patent: Paragraph 0706
[5] Patent: US2006/47126, 2006, A1. Location in patent: Page/Page column 31
