918516-27-5
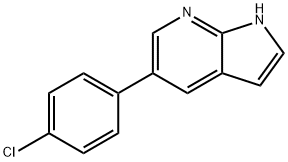 918516-27-5 结构式
918516-27-5 结构式
基本信息
VEMURAFENIB杂质1
5-(4-氯苯基)-7-氮杂吲哚
5-(4-氯苯基)-1H-吡咯并[2,3-B]吡啶
1H-Pyrrolo[2,3-b]pyridine, 5-(4-chlorophenyl)-
1H-Pyrrolo[2,3-b]pyridine, 5-(4-chlorophenyl)-###918516-27-5
物理化学性质
制备方法
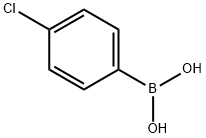
1679-18-1
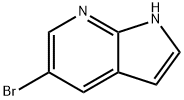
183208-35-7

918516-27-5
通用方法:步骤:将5-溴-7-氮杂吲哚(2g,10.2mmol,1.0当量)、K2CO3(2.8g,20.3mmol,2当量)和4-氯苯硼酸(1.8g,11.2mmol,1.1当量)悬浮于DME/H2O(30ml,4:1)混合溶剂中,并用氩气脱气。加入Pd(PPh3)4(587mg,508μmol,0.05当量),将反应混合物加热回流直至原料完全消耗。反应完成后,使溶液通过硅藻土垫过滤,用EtOAc稀释,并用水洗涤。合并有机层,用Na2SO4干燥,减压蒸发溶剂。粗产物通过快速色谱法(SiO2,正己烷/EtOAc 6:4)纯化。产量:2.23g,9.4mmol,92%(白色固体)。TLC:石油醚/EtOAc 1:1。1H NMR(DMSO-d6,200MHz,ppm):δ11.76(s,1H),8.51(d,J=2.1Hz,1H),8.20(d,J=1.9Hz,1H),7.72(d,J=8.5Hz,2H),7.57-7.43(m,3H),6.50(dd,J=3.2,1.7Hz,1H)。13C NMR(DMSO-d6,50MHz,ppm):δ148.2,141.4,138.0,131.7,128.9,128.6,127.1,126.9,126.1,117.7,100.2。
参考文献:
[1] Patent: WO2018/134254, 2018, A1. Location in patent: Page/Page column 35; 36; 79; 80
[2] Tetrahedron Letters, 2012, vol. 53, # 32, p. 4161 - 4165
[3] Patent: WO2015/75749, 2015, A1. Location in patent: Page/Page column 43; 44
[4] Journal of Medicinal Chemistry, 2017, vol. 60, # 23, p. 9470 - 9489
