940890-90-4
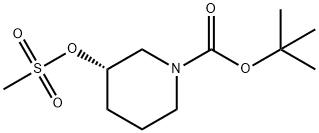 940890-90-4 结构式
940890-90-4 结构式
基本信息
(S)-1-BOC-3-甲磺酰氧基哌啶
(S)-3-(甲基磺酰氧基)哌啶-1-羧酸叔丁酯
(S)-3-((甲基磺酰基)氧基)哌啶-1-羧酸叔丁酯
(S)-1-BOC-3-(Methylsulfonyloxy)piperidine
(S)-1-(TERT-BUTOXYCARBONYL)PIPERIDIN-3-YL METHANESULFONATE
Tert-butyl (3S)-3-methylsulfonyloxypiperidine-1-carboxylate
1Piperidinecarboxylic acid, 3[(methylsulfonyl)oxy], 1,1dimeth
1-Piperidinecarboxylic acid, 3-[(Methylsulfonyl)oxy]-, 1,1-diMethylethyl ester, (3S)-
2-Methyl-2-propanyl (3S)-3-[(methylsulfonyl)oxy]-1-piperidinecarboxylate (S)tertButyl 3(methylsulfonyloxy)piperidine1carboxylate
制备方法

124-63-0
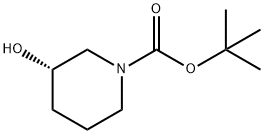
143900-44-1

940890-90-4
在氮气保护下,将(S)-1-叔丁氧羰基-3-羟基哌啶(10.0 g,49.7 mmol)和三乙胺(7.82 mL,54.7 mmol)溶解于二氯甲烷(60 mL)中,冷却至0℃。随后,缓慢滴加甲磺酰氯(4.23 mL,54.7 mmol),并在该温度下搅拌反应混合物1小时。反应完成后,用去离子水(60 mL)稀释反应液,并通过疏水性玻璃料过滤。有机层经减压浓缩,得到无色固体的叔丁基(3S)-3-(甲基磺酰氧基)哌啶-1-甲酸酯(13.9 g,49.7 mmol,收率100%)。产物经1H NMR(400 MHz,DMSO-d6)表征:δ(ppm)4.76-4.71(m,1H,CH),3.69-3.66(m,1H),3.65-3.60(m,1H),3.49-3.43(m,1H),3.37-3.31(m,1H),3.07(s,3H,CH3),2.02-1.96(m,1H),1.94-1.91(m,1H),1.87-1.80(m,1H),1.59-1.53(m,1H),1.48-1.45(m,9H)。
参考文献:
[1] Patent: WO2014/188173, 2014, A1. Location in patent: Paragraph 00224
[2] Patent: CN105399756, 2016, A. Location in patent: Paragraph 0152; 0167-0168
[3] MedChemComm, 2014, vol. 5, # 12, p. 1879 - 1886
[4] Patent: WO2017/134684, 2017, A2. Location in patent: Page/Page column 12
[5] Patent: CN103864673, 2016, B. Location in patent: Paragraph 0084; 0085