94284-66-9
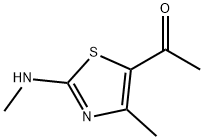 94284-66-9 结构式
94284-66-9 结构式
基本信息
1-[4-甲基-2-(甲氨基)-5-噻唑基]乙酮
1-(4-甲基-2-(甲氨基)噻唑-5-基)乙-1-酮
1-(4-METHYL-2-(METHYLAMINO)THIAZOL-5-YL)ETHANONE
1-[4-Methyl-2-(methylamino)-5-thiazolyl]ethanone
Ethanone, 1-[4-methyl-2-(methylamino)-5-thiazolyl]-
1-(4-Methyl-2-(methylamino)thiazol-5-yl)ethan-1-one
物理化学性质
制备方法
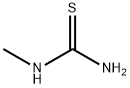
598-52-7
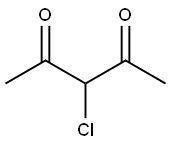
1694-29-7

94284-66-9
以N-甲硫脲和3-氯-2,4-戊二酮为原料合成1-(4-甲基-2-(甲氨基)噻唑-5-基)乙酮的一般步骤:将1-甲基硫脲(6.00 g,66.6 mmol)溶解于甲醇(130 mL)和吡啶(5 mL)的混合溶液中,并在冰浴中冷却。随后,向此冷却的溶液中缓慢滴加3-氯戊烷-2,4-二酮(8.96 mL,66.6 mmol)。将反应混合物逐渐升温至室温,并持续搅拌4小时。反应完成后,通过过滤收集生成的白色固体沉淀,并用乙醚(20 mL)洗涤,最终得到目标产物1-(4-甲基-2-(甲氨基)噻唑-5-基)乙酮(8.94 g,产率79%)。产物经1H-NMR(DMSO-d6)表征:δ 2.39(s,3H,CH3),2.49(s,3H,CH3),2.91(s,3H,CH3),9.12(s,1H,NH)。质谱(ESI)分析显示m/z [M+H]+计算值为171.06(C7H11N2OS+),实测值为171.06。
参考文献:
[1] European Journal of Medicinal Chemistry, 2017, vol. 139, p. 762 - 772
[2] Journal of Medicinal Chemistry, 2004, vol. 47, # 7, p. 1662 - 1675
[3] Journal of Medicinal Chemistry, 2010, vol. 53, # 11, p. 4367 - 4378
[4] Bioorganic and Medicinal Chemistry, 2011, vol. 19, # 10, p. 3135 - 3140
[5] Bioorganic and Medicinal Chemistry, 2011, vol. 19, # 24, p. 7349 - 7356