Alectinib Hydrochloride
|
|
|
- CAS-Nr.
- 1256589-74-8
- Englisch Name:
- Alectinib Hydrochloride
- Synonyma:
- Alectinib HCl;Alectinib Hydrochloride;CH-5428402;CPD0098(HCl);CH5424802 HCl;CH-5428402 HCl;Alectinib HCl salt;AF-802 Hydrochloride;RG-7853 Hydrochloride;CH5424802 Hydrochloride
- CBNumber:
- CB02570369
- Summenformel:
- C30H35ClN4O2
- Molgewicht:
- 519.09
- MOL-Datei:
- 1256589-74-8.mol
|
Alectinib Hydrochloride Eigenschaften
- storage temp.
- Store at -20°C
- Löslichkeit
- DMSO:3.5(Max Conc. mg/mL);6.74(Max Conc. mM)
- Aggregatzustand
- Solid
- Farbe
- White to Off-White
- Stabilität:
- Hygroscopic
Sicherheit
- Risiko- und Sicherheitserklärung
- Gefahreninformationscode (GHS)
| Bildanzeige (GHS) |

|
| Alarmwort |
Warnung |
| Gefahrenhinweise |
| Code |
Gefahrenhinweise |
Gefahrenklasse |
Abteilung |
Alarmwort |
Symbol |
P-Code |
| H341 |
Kann vermutlich genetische Defekte verursachen. |
Keimzellmutagenität |
Kategorie 2 |
Warnung |
|
P201,P202, P281, P308+P313, P405,P501 |
| H361 |
Kann vermutlich die Fruchtbarkeit beeinträchtigen oder das Kind im Mutterleib schädigen. |
Reproduktionstoxizität |
Kategorie 2 |
Warnung |
|
P201, P202, P281, P308+P313, P405,P501 |
| H373 |
Kann die Organe schädigen bei längerer oder wiederholter Exposition. |
Spezifische Zielorgan-Toxizität (wiederholte Exposition) |
Kategorie 2 |
Warnung |
|
P260, P314, P501 |
|
| Sicherheit |
| P201 |
Vor Gebrauch besondere Anweisungen einholen. |
| P202 |
Vor Gebrauch alle Sicherheitshinweise lesen und verstehen. |
| P260 |
Dampf/Aerosol/Nebel nicht einatmen. |
| P281 |
Vorgeschriebene persönliche Schutzausrüstung verwenden. |
| P308+P313 |
BEI Exposition oder falls betroffen: Ärztlichen Rat einholen/ärztliche Hilfe hinzuziehen. |
| P314 |
Bei Unwohlsein ärztlichen Rat einholen / ärztliche Hilfe hinzuziehen. |
| P405 |
Unter Verschluss aufbewahren. |
| P501 |
Inhalt/Behälter ... (Entsorgungsvorschriften vom Hersteller anzugeben) zuführen. |
|
Alectinib Hydrochloride Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Alectinib hydrochloride, developed by Chugai Pharmaceutical/
Hoffman-La Roche under the trade name Alecensa®, was approved
in Japan in April 2014 for the treatment of anaplastic lymphoma
kinase (ALK) fusion-gene positive, unresectable, advanced, or
recurrent non-small cell lung cancer (NSCLC). The compound is
a highly selective second-generation ALK inhibitor, and while
alectinib currently remains a focus of further development in Europe
and the U.S., the compound has been granted orphan drug designation
in Japan after showing a 93.5% objective response rate in
phase II clinical trials. In addition to providing rapid treatment
response time in a majority of patients, trials showed a 76%
2-year progression-free survival rate. Since the initial approval
of crizotinib—the first ALK inhibitor indicated for treatment of ALKrearranged
NSCLC —patients treated with crizotinib have shown
remarkable improvement as compared to treatment with other
chemotherapeutic methods,21 although drug resistance has shown
to be a major side effect of this therapy. Preliminary preclinical
and clinical studies of alectinib have shown significant promise
for overcoming drug resistance developed with other ALK
inhibitors.
Verwenden
CH5424802 Hydrochloride is a highly selective and potent anaplastic lymphoma kinase (ALK) inhibitor capable of blocking the resistant gatekeeper mutant, which results in reduced cell growth. Also is an intermediate of Alectinib (C183360), a highly selective and potent anaplastic lymphoma kinase (ALK) inhibitor capable of blocking the resistant gatekeeper mutant, which results in reduced cell growth.
Synthese
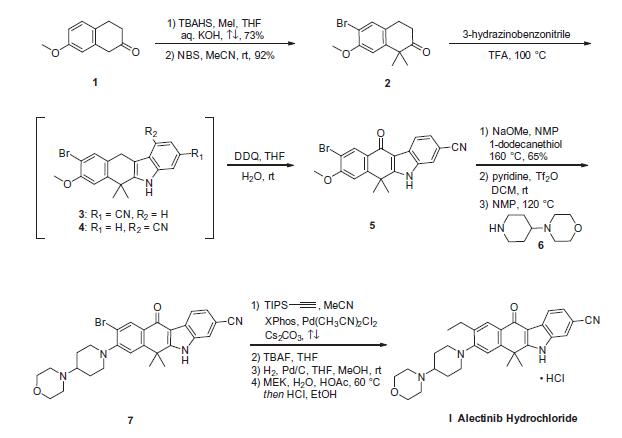
The synthetic route to alectinib as reported by Chugai
begins with 7-methoxy-2-tetralone (1). Bis-methylation
with tetrabutylammonium hydrogen sulfide (TBAHS)/aq KOH/MeI
followed by bromination with N-bromosuccinimide (NBS) provided
the bromo-tetralone 2 in 67% yield over the two steps. Further
reaction of 2 with 3-hydrazinobenzonitrile/trifluoroacetic acid (TFA) led to formation of the desired Fischer indole product,
albeit as a 1:1 mixture of regioisomers (3/4), which were carried
forward as a mixture to oxidation with 2,3-dichloro-5,6-dicyano-
1,4-benzoquinone (DDQ). It is important to note that although representative
procedures are published describing the conversion of
2 to alectinib (I), no yields were provided for these transformations.
Following oxidation, the desired product 5 could be isolated
as a single isomer via precipitation from the crude reaction mixture.
Installation of the 4-morpholino-piperidine moiety took place
in three transformations from 5, beginning with 1-dodecanethiol/
N-methyl-2-pyrrolidone (NMP)/NaOMe-facilitated methyl cleavage.
The corresponding phenol was then readily converted to the
triflate intermediate and displaced with 4-(piperidin-4-yl)morpholine
(6) at elevated temperature, providing intermediate 7. Crosscoupling
of the bromide 7 with ethynyl triisopropylsilane under
Pd-catalyzed cross-coupling conditions (Pd(CH3CN)2Cl2/2-dicyclohexylphosphino-
20,40,60-triisopropylbiphenyl (XPhos), reflux) followed
by cleavage of the resulting alkylsilane with
tetrabutylammonium fluoride (TBAF) yielded the ethynyl precursor
to alectinib. Hydrogenation of this unsaturated system under
standard conditions (H2, Pd/C) followed by HCl salt formation furnished
the final drug target alectinib hydrochloride (I).
IC 50
1.9 nM
Alectinib Hydrochloride Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Alectinib Hydrochloride Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global( 164)Lieferanten
- 9-ethyl-6,6-diMethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-5a,6,11,11a-tetrahydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride
- CH-5428402
- 9-Ethyl-6,11-dihydro-6,6-dimethyl-8-[4-(4-morpholinyl)-1-piperidinyl]-11-oxo-5H-benzo[b]carbazole-3-carbonitrile hydrochloride (1:1)
- AF-802 Hydrochloride
- CH5424802 Hydrochloride
- CH-5424802 Hydrochloride
- RG-7853 Hydrochloride
- RO-5424802 Hydrochloride
- CH 5424802,Alectinib(HCl)
- CH-5428402 HCl
- CH5424802 HCl (AF 802 HCl, Alectinib HCl)
- CH5424802 HCl salt, Alectinib HCl salt, AF802 HCl salt
- Alectinib HCl salt
- 9-ethyl-6,6-dimethyl-8-(4-morpholin-4-ylpiperidin-1-yl)-11-oxo-5H-benzo[b]carbazole-3-carbonitrile,hydrochloride
- Alectinib (CH5424802) HCl
- Alectinib (CH5424802) hydrochloride
- 9-ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile monohydrochloride monohydrate
- Alectinib Hydrochloride (Alecensa)
- 5H-Benzo[b]carbazole-3-carbonitrile, 9-ethyl-6,11-dihydro-6,6-dimethyl-8-[4-(4-morpholinyl)-1-piperidinyl]-11-oxo-, hydrochloride (1:1)
- CH5424802 HCl
- 9-ethyl-6,6-dimethyl-8-[4-(morpholin-4-yl)piperidin-1-yl]-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride
- Alectinib HCl (ALECENSA, AF-802, CH-5424802, RO-5424802)
- 9-Ethyl-6,6-dimethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-5a,6,11,11A-tetrahydro-5H-benzo[b]carbazole-3-carbonitrile HCL
- Alectinib HCl
- Alectinib Hydrochloride
- CPD0098(HCl)
- 9-Ethyl-6,11-dihydro-6,6-dimethyl-8-4-morpholin-4-yl-piperidin-1-yl-11-oxo-5H-benzobcarbazol-3-carbonitrile HCl
- Alectinib hydrochloride (JAN)
- 1256589-74-8
- C30H34N4O2HCl
- API
- 1256589-74-8

