Diethylenglykol Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
GERUCHLOSE, FARBLOSE, VISKOSE, HYGROSKOPISCHE FLüSSIGKEIT.
CHEMISCHE GEFAHREN
Reagiert sehr heftig mit starken Oxidationsmitteln. Feuer- und Explosionsgefahr. Greift einige Arten von Kunststoff an.
ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt.
MAK: 10 ppm; 44 mg/m? Spitzenbegrenzung: überschreitungsfaktor II(4) Schwangerschaft: Gruppe C (DFG 2007).
AUFNAHMEWEGE
Aufnahme in den Körper durch Verschlucken
INHALATIONSGEFAHREN
Beim Verdampfen bei 20°C tritt eine gesundheitsschädliche Kontamination der Luft nicht oder nur sehr langsam ein, viel schneller jedoch beim Versprühen oder Dispergieren.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: Möglich sind Auswirkungen auf die Nieren mit Nierenschäden. Möglich sind Auswirkungen auf das Zentralnervensystem und die Leber durch Verschlucken. Exposition durch Verschlucken kann zum Tod führen.
LECKAGE
Persönliche Schutzausrüstung: Atemschutzgerät mit Filter für organische Gase und Partikel entsprechend der Arbeitsplatzkonzentration des Stoffes. Ausgelaufene Flüssigkeit in abdichtbaren Behältern sammeln. Verschüttete Flüssigkeit mit viel Wasser wegspülen.
R-Sätze Betriebsanweisung:
R22:Gesundheitsschädlich beim Verschlucken.
S-Sätze Betriebsanweisung:
S46:Bei Verschlucken sofort ärztlichen Rat einholen und Verpackung oder Etikett vorzeigen.
Chemische Eigenschaften
Diethylene glycol is a clear colorless, odorless and stable oily liquid. It is also slightly viscous, noncorrosive and nonvolatile. Because of its ether and alcohol group, diethylene glycol exhibits chemical properties characteristic of both primary alcohols and ethers. Its boiling point is considerably higher than that of ethylene glycol, and its solvent is greater. Diethylene glycol is miscible with water, ethers, lower aliphatic alcohols, aldehydes and ketones and is partially soluble in benzene, carbon tetrachloride, monobenzene, orthodichlorobenzene and toluene. It dissolves many dyes, resins, oils, nitrocellulose and many organic substances. Because of its solvent power, low volatility and hygroscopicity, it is used in textile lubricants, cutting oils, dry cleaning soap, printing inks, steam-set inks, and nongrain wood stains. In the textile industry diethylene glycol is used as a conditioning agent for wool, rayon, and cotton. As a solvent for dyes it makes a valuable assistant in dyeing and printing. The high hygroscopicity of diethylene glycol makes it an efficient softening agent for tobacco, paper, synthetic sponges, glues and casein. Diethylene glycol is especially useful in the dehydration of natural gas. A mixture of diethylene glycol and monoethanolamine will remove moisture, hydrogen sulfide and carbon dioxide from natural gas.
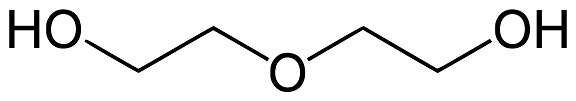
diethylene glycol structure
Vorbereitung Methode
Diethylene glycol is produced commercially as a by-product
of ethylene glycol production. It can also be produced
directly by reaction between ethylene glycol and ethylene
oxide .
Application
Diethylene glycol has many industrial uses. It is a component of antifreeze, brake fluids, cosmetics, inks, and drying agents, and it is used as a plasticizer. In antifreeze solution for sprinkler systems, water seals for gas tanks, etc. (water with 40% diethylene glycol freezes at -18°; with 50% at -28°); as lubricating and finishing agent for wool, worsted, cotton, rayon, and silk; as solvent for vat dyes; in composition corks, glues, gelatin, casein, and pastes to prevent drying out.
Allgemeine Beschreibung
Diethylene glycol appears as a colorless liquid. Denser than water. Contact may slightly irritate skin, eyes and mucous membranes. May be slightly toxic by ingestion. Used to make other chemicals.
Air & Water Reaktionen
Slightly soluble in water.
Reaktivität anzeigen
Diethylene glycol is incompatible with strong oxidizing agents. Diethylene glycol is also incompatible with strong bases. Diethylene glycol can react with sulfuric acid and other dehydrating agents, nitric acid, oxygen, hydrogen peroxide, perchloric acid and strong acids. Mixtures with sodium hydroxide decompose exothermically when heated to 446° F.
Health Hazard
Ingestion of large amounts may cause degeneration of kidney and liver and cause death. Liquid may cause slight skin irritation.
Brandgefahr
Diethylene glycol is combustible.
Toxikologie
The toxicity of diethylene glycol is similar to ethylene glycol and clearly is a CNS depressant. It has a low inhalation hazard because of its low vapor pressure; however, inhalation of the mist or aerosol is to be avoided. Workplace levels for vapors and aerosols cannot exceed 50 ppm. In case of accidental release of diethylene glycol, use of a full-face positive air pressure respirator is recommended. Even though the toxicokinetics in humans is not completely understood, its toxic nature is confirmed by animal studies. Several human cases were reported in the medical literature. Several children in Haiti died in 1995 and 1996 following the consumption of medication containing diethylene glycol. Similar other cases in children were reported in other countries as well. A 24-year-old man developed encephalopathy and rapidly became quadriplegic following ingestion of a solution containing diethylene glycol . Thus, the toxicity of diethylene glycol is well established.
Sicherheitsprofil
Moderately toxic to
humans by ingestion. Poison experimentally
by inhalation. Moderately toxic by ingestion
and intravenous routes. Questionable
carcinogen with experimental carcinogenic,tumorigenic, and teratogenic data. An eye
and human skin irritant. Combustible when
exposed to heat or flame; can react with
oxidning materials. To fight fire, use alcohol
foam, water, Con, dry chemical. Mixtures
with sodium hydroxide decompose
exothermically when heated to 230℃ and
release explosive hydrogen gas. When
heated to decomposition it emits acrid
smoke and irritating fumes. See also
GLYCOL ETHERS.
Carcinogenicity
Weil et al. , in their longterm
studies on rats of three different age levels, found only
one bladder tumor in those fed diets that contained 4%
diethylene glycol. This tumor was in a rat that also had
bladder stones . To clarify the question of the cause of
the tumor, Weil et al. implanted calcium oxalate
stones or glass beads into the bladders of rats. They found that
bladder tumors never developed without the presence of a
foreign body in the bladder. This led to the conclusion that
diethylene glycol essentially free of ethylene glycol is not a
primary carcinogen.
Environmental Fate
Diethylene glycol is metabolized by alcohol dehydrogenase to
toxic metabolites predominantly, HEAA and DGA. DEG can
cause an anion gap metabolic acidosis, cortical necrosis
resulting in permanent renal failure and neurotoxicity. DGA,
not HEAA, was recently identified as being the primary nephrotoxic agent causing proximal tubule cell death. The
neurotoxicity seen after DEG poisoning is only recently
described. The neurotoxicity is delayed and has cranial and
peripheral demyelinating sensorimotor polyneuropathy
pattern. The exact mechanism of the neurotoxicity remains
unclear and in the cases described in the literature, it appears to
be prolonged but does show evidence of reversibility.
Diethylenglykol Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Xylol, Isomerengemisch
polyurethane water-based emulsion finishes PU-II series
Natrium-4-(3,5-dichlor-4-oxocyclohexa-2,5-dienylidenamino)phenoxid
2-Chloro-4-dodecylphenol
Oxydiacetyldichlorid
2-Thiophenbuttersaeure
Pyren-1-buttersure
4-Methylmorpholin
Ursodeoxycholsure
4-Amino-2,6-dichlorphenol
Unsaturated polyester resin
2-(2-Hexyloxyethoxy)ethanol
polyurethae finishes PUC series
CSF series modified sacrylic binder
21-Iodo-16-methylpregna-1,4,9(11)-trien-17-ol-3,20-dione
AC anti-fungus leather finishing agent
Toluol
2-Ethoxyethyl ether
4-(4-Methoxyphenyl)buttersure
Oxydiethylendibenzoat
2-(2-Ethoxyethoxy)ethanol
6-Phenylhexanoic acid
Diamfenetid
thickening agent PAS
1-CHLORO-3-FLUOROISOPROPANOL
Benzol
16-Methylpregna-1,4,9(11)-trien-17-ol-3,20-dione
defoaming agent OTD
2,3-DIHYDROXYQUINOXALINE-6-CARBOXYLIC ACID
Ethandiol
BT modified acrylic resin binder series
weather-proof acrylic binder series
1-PYRENEDECANOIC ACID
4-Ethylmorpholin
Caseine
4-Benzylpiperidin
Diethylene Glycol Dibutyl Ether
1,3-Difluorpropan-2-ol
Morpholin
Disperse Yellow 126

