Phthalsureanhydrid Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
WEISSE GLäNZENDE KRISTALLE MIT CHARAKTERISTISCHEM GERUCH.
PHYSIKALISCHE GEFAHREN
Staubexplosion der pulverisierten oder granulierten Substanz in Gemischen mit Luft möglich.
CHEMISCHE GEFAHREN
Zersetzung bei Kontakt mitheißem Wasser unter Bildung von Phthalsäure. Reagiert mit starken Oxidationsmitteln, starken Säuren, starken Basen und Reduktionsmitteln. Reagiert sehr heftig beim Erhitzen mit Kupferoxid oder Natriumnitrit unter Explosionsgefahr. Greift viele Metalle in Gegenwart von Wasser an.
ARBEITSPLATZGRENZWERTE
TLV: 1 ppm; Sensibilisierung; Krebskategorie A4 (nicht klassifizierbar als krebserzeugend für den Menschen); (ACGIH 2005).
MAK: IIb (nicht festgelegt, aber Informationen vorhanden); Sensibilisierung der Atemwege; (DFG 2005).
AUFNAHMEWEGE
Aufnahme in den Körper durch Inhalation des Aerosols und durch Verschlucken.
INHALATIONSGEFAHREN
Eine gesundheitsschädliche Partikelkonzentration in der Luft kann beim Dispergieren schnell erreicht werden, vor allem als Pulver.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: Die Substanz reizt stark die Augen, die Haut und die Atemwege.
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Wiederholter oder andauernder Kontakt kann zu Hautsensibilisierung führen. Wiederholte oder andauernde Inhalation kann asthmatische Beschwerden (s. Anm.) hervorrufen.
LECKAGE
Verschüttetes Material in abgedeckten Behältern sammeln; falls erforderlich durch Anfeuchten Staubentwicklung verhindern. Reste sorgfältig sammeln. An sicheren Ort bringen. Persönliche Schutzausrüstung: Chemikalienschutzanzug mit umgebungsluftunabhängigem Atemschutzgerät.
R-Sätze Betriebsanweisung:
R22:Gesundheitsschädlich beim Verschlucken.
R37/38:Reizt die Atmungsorgane und die Haut.
R41:Gefahr ernster Augenschäden.
R42/43:Sensibilisierung durch Einatmen und Hautkontakt möglich.
S-Sätze Betriebsanweisung:
S23:Gas/Rauch/Dampf/Aerosol nicht einatmen(geeignete Bezeichnung(en) vom Hersteller anzugeben).
S24/25:Berührung mit den Augen und der Haut vermeiden.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S37/39:Bei der Arbeit geeignete Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S46:Bei Verschlucken sofort ärztlichen Rat einholen und Verpackung oder Etikett vorzeigen.
S22:Staub nicht einatmen.
Aussehen Eigenschaften
C8H4O3; 1,2-Benzoldicarbonsäureanhydrid. Farblose Kristalle mit schwach aromatischem Geruch.
Gefahren für Mensch und Umwelt
Reizt die Atmungsorgane, Augen und die Haut.
Verursacht beim Einatmen Husten und Atemnot.
Nicht mit Wasser, Alkalihydroxiden und Salpetersäure in Verbindung bringen.
LD
50 (oral, Ratte): 4020 mg/kg
Schutzmaßnahmen und Verhaltensregeln
Geeignete Schutzhandschuhe als kurzzeitiger Staubschutz.
Verhalten im Gefahrfall
Vorsichtig trocken aufnehmen. Der Entsorgung zuführen.
Wasser, Kohlendioxid, Pulver.
Brennbar. Gefahr einer Staubexplosion.
Erste Hilfe
Nach Hautkontakt: Mit reichlich Wasser abwaschen.
Nach Augenkontakt: Mit reichlich Wasser bei geöffnetem Lidspalt mindestens 10 Minuten ausspülen. Sofort Augenarzt hinzuziehen.
Nach Einatmen: Frischluft.
Nach Verschlucken: Reichlich Wasser trinken lassen. Erbrechen auslösen. Sofort Arzt hinzuziehen.
Nach Kleidungskontakt: Kontaminierte Kleidung entfernen.
Ersthelfer: siehe gesonderten Anschlag
Sachgerechte Entsorgung
Als feste Laborchemikalienabfälle.
Beschreibung
Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. This colourless solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics.

Phthalic anhydride is an important chemical intermediate in the plastics industry from which are derived numerous phthalate esters that function as plasticizers in synthetic resins. Phthalic anhydride itself is used as a monomer for synthetic resins such as glyptal, the alkyd resins, and the polyester resins.
Phthalic anhydride is also used as a precursor of anthraquinone, phthalein, rhodamine, phthalocyanine, fluorescein, and xanthene dyes.
Phthalic anhydride is used in the synthesis of primary amines, the agricultural fungicide phaltan, and thalidomide. Other reactions with phthalic anhydride yield phenolphthalein, benzoic acid, phthalylsulfathiazole (an intestinal antimicrobial agent), and orthophthalic acid.
Chemische Eigenschaften
Phthalic Anhydride is moderately flammable, white solid (flake) or a clear, colorless, mobile liquid (molten) Characteristic, acrid, choking odor. It is very slightly soluble in H2O, soluble in alcohol, and slightly soluble in ether.
Physikalische Eigenschaften
Colorless to pale cream crystals with a characteristic, choking odor. Moisture sensitive. Odor
threshold concentration is 53 ppb (quoted, Amoore and Hautala, 1983).
Definition
ChEBI: Phthalic anhydride is the cyclic dicarboxylic anhydride that is the anhydride of phthalic acid. It has a role as an allergen. It is a cyclic dicarboxylic anhydride and a member of 2-benzofurans.
synthetische
The most important modifying component used in the
manufacture of linear unsaturated polyesters is phthalic anhydride. The
anhydride is generally obtained by the oxidation of o-xylene:
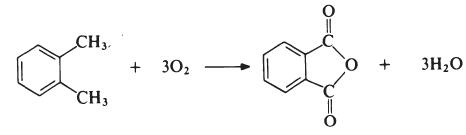
The reaction is carried out in the vapour phase by passing a mixture of
o-xylene and air over a catalyst such as vanadium pentoxide supported on
silica and promoted with titanium dioxide at about 400??C. The exit gases are
cooled and the phthalic anhydride is collected and purified by distillation
under reduced pressure.
Allgemeine Beschreibung
A colorless to white lustrous solid in the form of needles with a mild distinctive odor. Moderately toxic by inhalation or ingestion and a skin irritant. Melting point 64°F Flash point 305°F. Forms a corrosive solution when mixed with water. Used in the manufacture of materials such as artificial resins.
Air & Water Reaktionen
Reacts, usually slowly with water to form phthalic acid and heat [Merck 11th ed. 1989]. The phthalic acid is somewhat soluble in water.
Reaktivität anzeigen
Phthalic anhydride reacts exothermically with water. The reactions are sometimes slow, but can become violent when local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases. Undergoes exothmeric nitration with fuming nitric acid-sulfuric acid and may give mixtures of the potentially explosive phthaloyl nitrates or nitrites or their nitro derivatives [Chem. & Ind. 20:790. 1972]. Phthalic anhydride reacts violently with CuO at elevated temperatures [Park, Chang-Man, Richard J. Sheehan. hthalic Acids and Other Benzenepolycarboxylic Acids Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2005]. Mixtures of Phthalic anhydride and anhydrous CO2 explode violently if heated [eaflet No. 5, Inst. of Chem., London, 1940].
Health Hazard
Solid irritates skin and eyes, causing coughing and sneezing. Liquid causes severe thermal burns.
Brandgefahr
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Pharmazeutische Anwendungen
Phthalic anhydride reacted with cellulose acetate forms cellulose acetate phthalate (CAP), a common enteric coating excipient that has also been shown to have antiviral activity. Phthalic anhydride is a degradation product of CAP.
Kontakt-Allergie
Phthalic anhydride is used in the manufacture of unsaturated
polyesters and as a curing agent for epoxy resins.
When used as a pigment, it can be responsible for sensitization
in ceramic workers. Phthalic anhydride per se is
not responsible for the sensitization to the resin used in
nail varnishes phthalic anhydride/trimellitic anhydride/
glycols copolymer.
Sicherheitsprofil
Poison by ingestion.
Experimental teratogenic effects. A
corrosive eye, skin , and mucous membrane
irritant. A common air contaminant.
Combustible when exposed to heat or flame; can react with oxidzing materials.
Moderate explosion hazard in the form of
dust when exposed to flame. The
production of ths material has caused many
industrial explosions. Mixtures with copper
oxide or sodium nitrite explode when
heated. Violent reaction with nitric acid +
sulfuric acid above 80℃. To fight fire, use
CO2, dry chemical. Used in plasticizers,
polyester resins, and alkyd resins, dyes, and
drugs. See also ANHYDRIDES.
mögliche Exposition
Phthalic anhydride is used in plasticizers; in the manufacture of phthaleins; benzoic acid; alkyd and polyester resins; synthetic indigo; and phthalic acid;which is used as a plasticizer for vinyl resins. To a lesser extent, it is used in the production of alizarin, dye, anthranilic acid; anthraquinone, diethyl phthalate; dimethyl phthalate; erythrosine, isophthalic acid; methylaniline, phenolphthalein, phthalamide, sulfathalidine, and terephthalic acid. It has also found uses as a pesticide intermediate.
Versand/Shipping
UN2214 Phthalic anhydride with>.05 % maleic anhydride, Hazard class: 8; Labels: 8-Corrosive material.
läuterung methode
Distil the anhydride under reduced pressure. Purify it from the acid by extracting with hot CHCl3, filtering and evaporating. The residue is crystallised from CHCl3, CCl4 or *benzene, or sublimed. Fractionally crystallise it from its melt. Dry it under vacuum at 100o. [Saltiel J Am Chem Soc 108 2674 1986, Beilstein 17/11 V 253.]
Inkompatibilitäten
Dust forms an explosive mixture with air. Phthalic anhydride reacts exothermically with water. The reactions are sometimes slow, but can become violent when local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases. Converted to phthalic acid in hot water. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. caustics, ammonia, amines, water. Reacts violently with copper oxide or sodium nitrite 1 heat.
Waste disposal
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Phthalsureanhydrid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Diallylphthalat
Kaliumhydrogenphthalat
1,2,5-OXADIAZOLE-3-CARBOXYLIC ACID
N-(Hydroxymethyl)phthalimide
N-(Tetrahydro-2,6-dioxo-2H-pyran-3-yl)phthalimid
3',6'-Bis(diethylamino)spiro-(isobenzofuran-1(3H),9'-(9H)xanthen)-3-on
Alkyd resin insulating paint
2',7'-Dibrom-3',6'-dihydroxyspiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-on
DI-ISO-DECYL PHTHALATE
2-BENZOYLBENZOYL CHLORIDE
N-Phenylphthalimid
o-Phenethylbenzoesure
1-CHLORO-4-METHOXYPHTHALAZINE
2-Chloranthrachinon
2-[4-Chlor-3-(chlorsulfonyl)benzoyl]benzoesure
Diphenylphthalat
Dipentylphthalat
Amino resin varnish
2,3,4,5-Tetrafluorobenzoic acid
Dinonylphthalat
2-Benzoylbenzoesure
2,3-DIHYDRO-2-PHENYL-1H-ISOINDOL-1-OXO-ISOINDOLINE
1,2-Benzoldicarbonsure, Di-C8-10-verzweigte Alkylester, C9-reich
2-Methylanthrachinon
Diisobutylphthalat
Dicyclohexylphthalat
Indobufen
Diisooctylphthalat
2-Ethylanthrachinon
DI-N-OCTYL PHTHALATE
(Z)-Cycloocten
N-(Trichlormethylthio)phthalimid
Fluorescein
Bis(1-methylheptyl)phthalat
N-2-Bromethylphthalimid
2-(MORPHOLINE-4-CARBONYL)-BENZOIC ACID
3-Benzylidenphthalid
3-METHYL-1,2,5-OXADIAZOLE
12H-Phthaloperin-12-on
Didecylphthalat

