Azilsartan kaMedoxoMil
|
|
|
- CAS-Nr.
- 863031-24-7
- Englisch Name:
- Azilsartan kaMedoxoMil
- Synonyma:
- TAK 491 kamedoxomil;TAK-491 monopotassium;Azilsartan kaMedoxoMil;Azilsartan KaModoxoMil;Azilsartan KaMedoximil;Azilsartan Mehtyl ester;Azilsartan MedoxMil PotassuiM;Azilsartan Medoxomil Potassium;Azilsartan MedoxiMil PotassiuM;Azilsartan MedoxoMil PotassiuM salt
- CBNumber:
- CB92549391
- Summenformel:
- C30H25KN4O8
- Molgewicht:
- 608.65
- MOL-Datei:
- 863031-24-7.mol
|
Azilsartan kaMedoxoMil Eigenschaften
- Schmelzpunkt:
- 193 - 195°C
- storage temp.
- Refrigerator, Under inert atmosphere
- Löslichkeit
- DMSO (Slightly), Methanol (Slightly)
- Aggregatzustand
- Solid
- Farbe
- White to Off-White
Sicherheit
- Risiko- und Sicherheitserklärung
- Gefahreninformationscode (GHS)
| Bildanzeige (GHS) |

|
| Alarmwort |
Warnung |
| Gefahrenhinweise |
| Code |
Gefahrenhinweise |
Gefahrenklasse |
Abteilung |
Alarmwort |
Symbol |
P-Code |
| H302 |
Gesundheitsschädlich bei Verschlucken. |
Akute Toxizität oral |
Kategorie 4 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P270, P301+P312, P330, P501 |
| H315 |
Verursacht Hautreizungen. |
Hautreizung |
Kategorie 2 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P280, P302+P352, P321,P332+P313, P362 |
| H319 |
Verursacht schwere Augenreizung. |
Schwere Augenreizung |
Kategorie 2 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P280, P305+P351+P338,P337+P313P |
| H335 |
Kann die Atemwege reizen. |
Spezifische Zielorgan-Toxizität (einmalige Exposition) |
Kategorie 3 (Atemwegsreizung) |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
|
|
| Sicherheit |
| P261 |
Einatmen von Staub vermeiden. |
| P280 |
Schutzhandschuhe/Schutzkleidung/Augenschutz tragen. |
| P301+P312 |
BEI VERSCHLUCKEN: Bei Unwohlsein GIFTINFORMATIONSZENTRUM/Arzt/... (geeignete Stelle für medizinische Notfallversorgung vom Hersteller/Lieferanten anzugeben) anrufen. |
| P302+P352 |
BEI BERÜHRUNG MIT DER HAUT: Mit viel Wasser/... (Hersteller kann, falls zweckmäßig, ein Reinigungsmittel angeben oder, wenn Wasser eindeutig ungeeignet ist, ein alternatives Mittel empfehlen) waschen. |
| P305+P351+P338 |
BEI KONTAKT MIT DEN AUGEN: Einige Minuten lang behutsam mit Wasser spülen. Eventuell vorhandene Kontaktlinsen nach Möglichkeit entfernen. Weiter spülen. |
|
Azilsartan kaMedoxoMil Chemische Eigenschaften,Einsatz,Produktion Methoden
Definition
ChEBI: An organic potassium salt that is the monopotassium salt of azilsartan medoxomil. A prodrug for azilsartan, it is used for treatment of hypertension.
Clinical Use
Azilsartan kamedoxomil, developed by Takeda Pharmaceuticals,
was approved for the treatment of hypertension and launched in
the U.S. under the brand name Edarbi. Edarbi is a prodrug that
undergoes rapid hydrolysis to liberate azilsartan, the active ingredient
(TAK-536, 39). As the 8th angiotensin receptor
blocker (ARB) to enter the world market, azilsartan kamedoxomil can function as monotherapy or in combination with other antihypertensive
agents. In several clinical studies, monotherapeutic azilsartan
kamedoxomil showed superior antihypertensive activity and a favorable safety/tolerability profile in patients compared
with other established therapeutics, including valsartan, olmesartan
medoxomil, candesartan, and telmisartan.In late 2011,Takeda announced that FDA also approved the fixed-dose combination
tablet of azilsartan kamedoxomil with chlorthalidone under
the trade name of Edarbyclor.
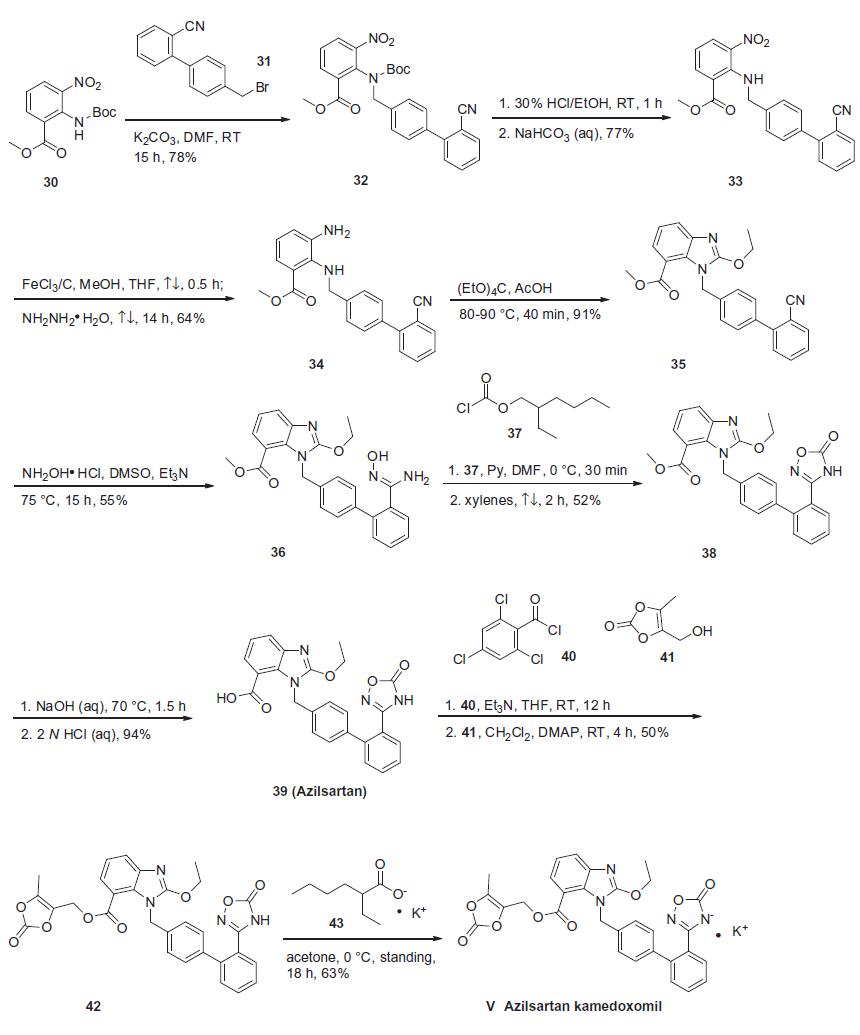
Synthese
Based on the synthesis of azilsartan, the process-scale approach to azilsartan kamedoxomil is
described in the scheme. The synthesis started with commercially
available methyl 2-[(tert-butoxycarbonyl)amino]-3-nitrobenzoate
(30), which can also be prepared by several different routes.41,42
Alkylation of 30 with diaryl bromide 31 gave benzylamine 32 in
78% yield, which was followed by deprotection with 30% ethanolic
HCl and alkalinization to produce amine 33 in 77% yield. The nitro
group within 33 was reduced with hydrazine hydrate and a catalytic
amount of ferric chloride to afford 2,3-diaminobenzoate 34
in 64% yield. Ring formation was achieved by treatment of 34 with
tetraethoxymethane and acetic acid to produce benzimidazole 35
in 91% yield.37 The addition of hydroxylamine to the cyano group
of 35 provided amidoxime 36 in 55% yield, which underwent
immediate cyclization upon treatment with 2-ethylhexyl chloroformate
37 in refluxing xylenes to give oxadiazolone 38 in 52%
yield. Hydrolysis of 38 gave azilsartan (39, TAK-536) in 94%
yield. In the presence of perchlorobenzoyl chloride 40 and triethylamine,
carboxylic acid 39 was converted to the mixed acid
anhydride intermediate, which when condensed with alcohol 41
furnished benzoate 42 in 50% yield. Salt preparation of 42 was
accomplished with potassium 2-ethylhexyl carboxylate 43 affording
azilsartan kamedoxomil (V) in 63% yield.
Azilsartan kaMedoxoMil Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Azilsartan kaMedoxoMil Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global( 163)Lieferanten
- Azilsartan kaMedoxoMil
- 1-[[2'-(2,5-Dihydro-5-oxo-1,2,4-oxadiazol-3-yl)[1,1'-biphenyl]-4-yl]methyl]-2-ethoxy-1H-benzimidazole-7-carboxylic acid (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl ester potassium salt
- Azilsartan MedoxoMil PotassiuM salt
- Azilsartan MedoxoMil (MonopotassiuM salt)
- Azilsartan MedoxoMil (MonopotassiuM)
- Azilsartan MedoxiMil PotassiuM
- Azilsartan KaModoxoMil
- Azilsartan kaMedoxoMil (with 5 ints.)
- Azilsartan MedoxMil PotassuiM
- 1H-BenziMidazole-7-carboxylic acid, 1-[[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)[1,1'-biphenyl]-4-yl]Methyl] -2-ethoxy-, (5-Methyl-2-oxo-1,3-dioxol-4-yl)Methyl ester, potassiuM salt
- Azilsartan Medoxomil Potassium
- Azilsartan Mehtyl ester
- Azilsartan Impurity 50 Potassium Salt
- Azilsartan medoxomil monopotassium (TAK 491)
- Azilsartan KaMedoximil
- Azilsartan Medoxomil Potassium Crude
- Azilsartan Impurity 45 Potassium Salt
- (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-ethoxy-1-((2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)-[1,1'-biphenyl]-4-yl)methyl)-1H-benzo[d]imidazole-7-carboxylate, potassium salt
- TAK 491 kamedoxomil
- TAK-491 monopotassium
- Azilsartan Impurity 60 Potassium Salt
- 863031-24-7
- C30H23KN4O8
- C30H23N4O8K
- Azilsartan Kamedoxomil
- 863031-24-7

