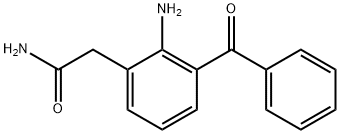
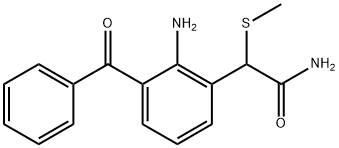
Nepafenac
- CAS No.
- 78281-72-8
- Chemical Name:
- Nepafenac
- Synonyms
- Nevanac;CS-328;AL 6515;AHR 9434;Nepafenac;Nepafanac;Nepafenac>Nepafenac API;NEPAFENAC STD;Nepafenac - WS
- CBNumber:
- CB01116872
- Molecular Formula:
- C15H14N2O2
- Molecular Weight:
- 254.28
- MDL Number:
- MFCD08067732
- MOL File:
- 78281-72-8.mol
- MSDS File:
- SDS
| Melting point | 177-181°C |
|---|---|
| Boiling point | 562.5±50.0 °C(Predicted) |
| Density | 1.249±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: ≥5mg/mL |
| pka | 16.09±0.40(Predicted) |
| form | powder |
| color | faint yellow to dark yellow |
| Water Solubility | 0.014 mg/mL in water |
| Merck | 14,6469 |
| InChI | InChI=1S/C15H14N2O2/c16-13(18)9-11-7-4-8-12(14(11)17)15(19)10-5-2-1-3-6-10/h1-8H,9,17H2,(H2,16,18) |
| InChIKey | QEFAQIPZVLVERP-UHFFFAOYSA-N |
| SMILES | C1(CC(N)=O)=CC=CC(C(=O)C2=CC=CC=C2)=C1N |
| CAS DataBase Reference | 78281-72-8(CAS DataBase Reference) |
| FDA UNII | 0J9L7J6V8C |
| ATC code | S01BC10 |
Nepafenac price More Price(36)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML0288 | Nepafenac ≥98% (HPLC) | 78281-72-8 | 10mg | $81.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML0288 | Nepafenac ≥98% (HPLC) | 78281-72-8 | 50mg | $316 | 2024-03-01 | Buy |
| TCI Chemical | N0932 | Nepafenac >98.0%(HPLC)(T) | 78281-72-8 | 200mg | $168 | 2024-03-01 | Buy |
| TCI Chemical | N0932 | Nepafenac >98.0%(HPLC)(T) | 78281-72-8 | 1g | $589 | 2024-03-01 | Buy |
| Cayman Chemical | 23700 | Nepafenac ≥98% | 78281-72-8 | 10mg | $44 | 2024-03-01 | Buy |
Nepafenac Chemical Properties,Uses,Production
Description
Nepafenac, launched by Alcon Laboratories, is a topical ophthalmic medication indicated for the treatment of ocular pain and inflammation associated with cataract surgery. Nepafenac is a prodrug of amfenac, which is an NSAID and a potent non-selective inhibitor of COX-1 (IC50=0.25 μM)) and COX-2 (IC50=0.15μM). Nepefenac itself exhibits only weak activity against COX-1 (IC50=64.3μM). Amfenac (Fenazox) has been marketed in Japan since 1986 for the treatment of rheumatoid arthritis, post-surgical pain, and inflammation. With most NSAIDs that are currently being used as topical ophthalmic agents, the maximum drug concentration is achieved on the ocular surface, with progressively lower concentrations in the cornea, aqueous humor, vitreous, and retina. Nepafenac has been found to have a penetration coefficient that is 4-28 times greater than that achieved with conventional NSAIDs such as diclofenac, bromofenac, and ketorolac. In addition, the bioconversion of nepefenac to amfenac is primarily mediated by ocular tissue hydrolases, specifically in the iris, ciliary body, retina, and choroid. The enhanced permeability of nepefenac combined with rapid bioactivation in the ocular tissue translates into superior anti-inflammatory efficacy at the target sites.
Chemical Properties
Nepafenac is a light Yellow Solid or powder. It is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF).
Originator
AH Robins (US)
Uses
Nepafenac is a non-steroidal anti-inflammatory drug (NSAID), usually sold as a prescription eye drop. It reduces pain and inflammation in the eyes. Nepafenac ophthalmic is used to reduce pain and swelling after cataract surgery.
Definition
ChEBI: Nepafenac is a monocarboxylic acid amide that is amfenac in which the carboxylic acid group has been converted into the corresponding carboxamide. It is a prodrug for amfenac, used in eye drops to treat pain and inflammation following cataract surgery. It has a role as a prodrug, a cyclooxygenase 2 inhibitor, a cyclooxygenase 1 inhibitor, a non-steroidal anti-inflammatory drug and a non-narcotic analgesic.
brand name
Nevanac (Alcon).
Biochem/physiol Actions
Nepafenac is a NSAID (nonsteroidal anti inflammatory drug) that is routinely used in opthamology to control pain following cataract surgery.
Clinical Use
Nepafenac is a novel ophthalmic non-steroidal anti-inflammatory drug (NSAID), for the treatment of eye pain and inflammation caused by cataract surgery, compared with traditional NSAIDs, chemical structure of Nepafenac is conducive to make it rapidly penetrate the cornea and distribute to its target site, which is helpful to reduce the accumulation of the drug in the corneal surface and to reduce the incidence of complications of the eye surface, it has many advantages such as infiltration, targeting strong, little toxic side effects and so on.
August 19, 2005 ,the US Food and Drug Administration (FDA) approved nepafenac ophthalmic suspension for the treatment of cataract surgery-related pain and inflammation, it is the first ophthalmic NSAID prodrug formulation approved for marketing.
Nepafenac after ocular administration, can rapidly pass through the cornea , and under the action of eye tissue hydrolytic enzymes,it can become into ammonia diclofenac (a kind of NSAID); and ammonia diclofenac by inhibiting prostaglandin H synthase ( cyclooxygenase), can block prostaglandin synthesis to play its role as an anti-inflammatory analgesic. As is known, prostaglandin is one of the media causing ocular inflammation it can lead to blood-aqueous barrier crash, vasodilatation, increased vascular permeability and leukocyte chemotaxis, etc. In addition, prostaglandins can also control contraction of the iris sphincter through non-cholinergic mechanism which can trigger the miosis reaction during eye surgery and after surgery. After ocular administration of NSAIDs, it can inhibit prostaglandins synthesis in the iris, ciliary body and conjunctiva, so people can prevent eye inflammation, and reduce the associated pain.
The above information is edited by the chemicalbook of Tian Ye.
Synthesis
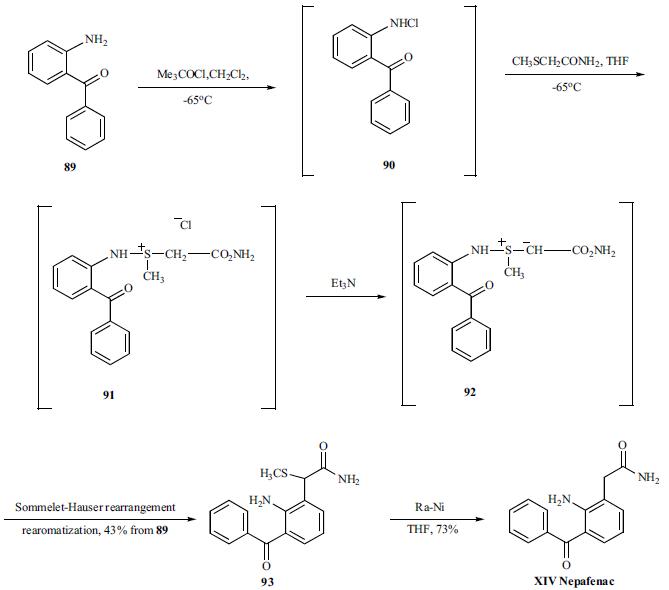
The synthesis of nepafenac started with commercially available 2-amino-benzophenone (89). Compound 89 was reacted with t-butyl hypochrite at ¨C 65??C in DCM to give a mono-N-chloroaniline (90) which was subsequently treated with methylthioacetamide in THF at ¨C65??C in the same pot to give an aza-sulfonium salt 91 as a solid. Compound 91 was slurred in DCM and triethylamine was added to give sulfer ylide 92 intermediate which under-went a Sommelet-Hauser type rearrangement to give compound 93 after re-aromatization of the intermediate cyclohexadienone imine. Compound 93 was finally reduced with Raney nickel to give nepafenac (XIV) in 73% yield as yellow needles.
Veterinary Drugs and Treatments
Nepafenac is a nonsteroidal anti-inflammatory and analgesic prodrug. After topical ocular dosing, nepafenac penetrates the cornea and is converted by ocular tissue hydrolases to amfenac, a nonsteroidal anti-inflammatory drug. Amfenac is thought to inhibit the action of prostaglandin H synthase (cyclooxygenase), an enzyme required for prostaglandin production. Nepafenac is indicated for the treatment of pain and inflammation associated with cataract surgery.
Clinical claims and research
In preclinical models, a single topical ocular dose of nepefenac (0.1%) inhibits prostaglandin synthesis in the iris/ciliary body by 85–95% for more than 6 h, and in the retina/choroid by 55% for up to at least 4 h. By comparison, diclofenac (0.1%) shows 100% inhibition of prostaglandin synthesis in the iris/ciliary body for only 20 min, with 75% recovery observed within 6 h. Diclofenac’s inhibition of prostaglandin synthesis in the retina/choroids is minimal. The recommended dose of nepafenac ophthalmic suspension is one drop in the affected eye(s) three times daily beginning one day prior to cataract surgery, continued on the day of surgery and through the first two weeks of the postoperative period. Although the drug is applied topically, low but quantifiable plasma concentrations of nepefenac and amfenac are observed in majority of the subjects following t.i.d. dosing of nepefenac ophthalmic solution. The clinical significance of the systemic absorption of nepefenac after ophthalmic administration is unknown. The efficacy of nepafenac was demonstrated in two placebo-controlled clinical studies involving over 680 patients. Nepafenac suspension was dosed three times daily, beginning one day prior to cataract surgery, continuing on the day of surgery, and for 14 days postoperatively.
Mode of action
Nepafenac is a non-steroidal anti-inflammatory and analgesic prodrug. After topical ocular dosing, nepafenac penetrates the cornea and is converted by ocular tissue hydrolases to amfenac, a nonsteroidal anti-inflammatory drug. Amfenac inhibits the action of prostaglandin H synthase (cyclooxygenase), an enzyme required for prostaglandin production.
References
[1] bucolo c1, marrazzo g, platania cb, romano gl, drago f, salomone s. effects of topical indomethacin, bromfenac and nepafenac on lipopolysaccharide-induced ocular inflammation. j pharm pharmacol. 2014 jul;66(7):954-60.
[2] marshall jc1, caissie al, cruess sr, cools-lartigue j, burnier mn jr. the effects of a cyclooxygenase-2 (cox-2) expression and inhibition on human uveal melanoma cell proliferation and macrophage nitric oxide production. j carcinog. 2007 nov 27;6:17.
Nepafenac Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shandong Hengshannuode Pharmaceutical Technology Co., Ltd. | +8615065888978 | admin@hsnordpharma.com | China | 92 | 58 |
| Tianjin Kilo Pharmaceutical Sci-Tech Co., Ltd | +86-02223869539 +86-15560057295 | trade.kilopharma@foxmail.com | China | 27 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| Nanjing Finetech Chemical Co., Ltd. | 025-85710122 17714198479 | sales@fine-chemtech.com | CHINA | 885 | 55 |
View Lastest Price from Nepafenac manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Nepafenac
78281-72-8
|
US $0.00 / Kg/Bag | 2Kg/Bag | 98.5% up / EP | 20 tons | Sinoway Industrial co., ltd. | |
 |
2023-09-08 | Nepafenac
78281-72-8
|
US $50.00 / kg | 1kg | 99% | 10000tons | Anhui Ruihan Technology Co., Ltd | |
 |
2023-06-15 | Nepafenac
78281-72-8
|
US $4126.00 / g/Bag | 500g | 99% | 100kg | Baoji Guokang Bio-Technology Co., Ltd. |