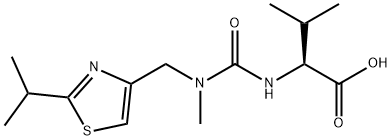
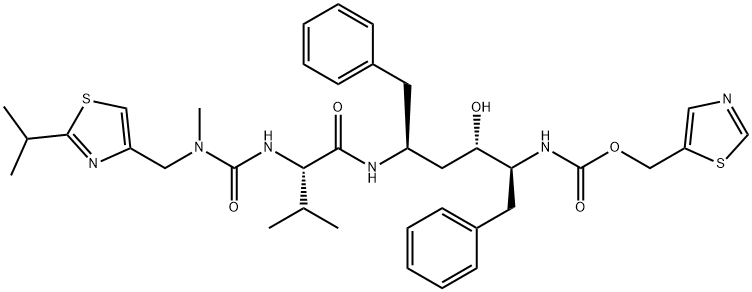
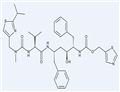
Ritonavir
- CAS No.
- 155213-67-5
- Chemical Name:
- Ritonavir
- Synonyms
- Ritonavi;THIAZOL-5-YL-METHANOL;Ritonavir for peak identification CRS;Norvir;RARECHEM AL BD 1261;thiazol-5-ylMethyl ((2S,3S,5R)-3-hydroxy-5-((S)-2-(3-((2-isopropylthiazol-4-yl)Methyl)-3-Methylureido)-3-MethylbutanaMido)-1,6-diphenylhexan-2-yl)carbaMate;thiazol-5-ylmethyl ((2S,3S,5S)-3-hydroxy-5-((S)-2-(3-((2-isopropylthiazol-4-yl)methyl)-3-methylureido)-3-methylbutanamido)-1,6-diphenylhexan-2-yl)carbamate;1,3-Thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[[(2S)-3-methyl-2-[[methyl-[(2-propan-2-yl-1,3-thiazol-4-yl)methyl]carbamoyl]amino]butanoyl]amino]-1,6-diphenylhexan-2-yl]carbamate;1,3-thiazol-5-ylmethyl n-[(2s,3s,5r)-3-hydroxy-5-[[(2s)-3-methyl-2-[[methyl-[(2-propan-2-yl-1,3-thiazol-4-yl)methyl]carbamoyl]amino]butanoyl]amino]-1,6-diphenyl-hexan-2-yl]carbamate;2,4,7,12-Tetraazatridecan-13-oic acid, 10-hydroxy-2-methyl-5-(1-methylethyl)-1-[2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-, 5-thiazolylmethyl ester, [5S-(5R*,8R*,10R*,11R*)]-
- CBNumber:
- CB0119429
- Molecular Formula:
- C37H48N6O5S2
- Molecular Weight:
- 720.94
- MDL Number:
- MFCD00927142
- MOL File:
- 155213-67-5.mol
- MSDS File:
- SDS
| Melting point | 120-122°C |
|---|---|
| Boiling point | 947.0±65.0 °C(Predicted) |
| Density | 1.239±0.06 g/cm3(Predicted) |
| Flash point | 2℃ |
| storage temp. | room temp |
| solubility | DMSO: soluble10mg/mL (clear solution, warmed) |
| form | powder |
| pka | 11.47±0.46(Predicted) |
| color | white to beige |
| Water Solubility | 5mg/L(ms) |
| Merck | 14,8238 |
| BCS Class | 2,4 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 2 months. |
| InChIKey | NCDNCNXCDXHOMX-XGKFQTDJSA-N |
| CAS DataBase Reference | 155213-67-5(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | Norvir; ritonavir |
| FDA UNII | O3J8G9O825 |
| NCI Drug Dictionary | Norvir |
| ATC code | J05AE03 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H312+H332 | |||||||||
| Precautionary statements | P261-P264-P280-P301+P312-P302+P352+P312-P304+P340+P312 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36/38-20/21/22-41 | |||||||||
| Safety Statements | 26-37/39-36/37-39 | |||||||||
| RIDADR | UN 1648 3 / PGII | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | XA5310000 | |||||||||
| HS Code | 29341000 | |||||||||
| NFPA 704 |
|
Ritonavir price More Price(52)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR1734 | Ritonavir | 155213-67-5 | 1g | $124 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1604803 | Ritonavir United States Pharmacopeia (USP) Reference Standard | 155213-67-5 | 200mg | $436 | 2024-03-01 | Buy |
| Cayman Chemical | 13872 | Ritonavir ≥98% | 155213-67-5 | 10mg | $28 | 2024-03-01 | Buy |
| Cayman Chemical | 13872 | Ritonavir ≥98% | 155213-67-5 | 50mg | $111 | 2024-03-01 | Buy |
| Sigma-Aldrich | Y0000714 | Ritonavir European Pharmacopoeia (EP) Reference Standard | 155213-67-5 | y0000714 | $220 | 2024-03-01 | Buy |
Ritonavir Chemical Properties,Uses,Production
Description
Ritonavir (brand name: Norvir) was launched in Germany, the UK and US for treatment of advanced HIV in combination with antiretroviral nucleoside analogs in a record 72 days by the FDA. It is an inhibitor of HIV aspartic protease which is critical in the processing of a propeptide into the gag, gag-pol gene products and the protease itself. This inhibition results in the release of non-infectous immature virus particles. It is greater than 500-fold more selective for viral aspartic protease than the human version, has good oral bioavailability and may increase the bioavailability of other protease inhibitors. Ritonavir was able to increase the CD4 and CD8 lymphocyte count as well as reduce viral RNA. It is more potent than saqunavir and comparible in potency to zidovudine and lamivudine.
Chemical Properties
Ritonavir is a white-to-light-tan powder with a bitter metallic taste. It is freely soluble in methanol and ethanol, soluble in isopropanol and practically insoluble in water. It is an inhibitor of HIV protease with activity against the Human Immunodeficiency Virus (HIV).
Originator
Abbott (USA)
Uses
Ritonavir is indicated in combination with other antiretroviral agents for the treatment of HIV-1-infected patients. Ritonivir is an HIV protease inhibitor (EC50 = 25 nM) that also inhibits CYP3A4, the primary cytochrome P450 isoform that metabolizes protease inhibitors. Through CYP3A4 inhibition, low doses of ritonivir can reduce the metabolism of concomitantly administered protease inhibitiors, enhancing their bioavailability and efficacy.
Definition
ChEBI: Ritonavir is an L-valine derivative that is L-valinamide in which alpha-amino group has been acylated by a [(2-isopropyl-1,3-thiazol-4-yl)methyl]methylcarbamoyl group and in which a hydrogen of the carboxamide amino group has been replaced by a (2R,4S,5S)-4-hydroxy-1,6-diphenyl-5-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}hexan-2-yl group. A CYP3A inhibitor and antiretroviral drug from the protease inhibitor class used to treat HIV infection and AIDS, it is often used as a fixed-dose combination with another protease inhibitor, lopinavir. Also used in combination with dasabuvir sodium hydrate, ombitasvir and paritaprevir (under the trade name Viekira Pak) for treatment of chronic hepatitis C virus genotype 1 infection as well as cirrhosis of the liver. It has a role as an antiviral drug, a HIV protease inhibitor, an environmental contaminant and a xenobiotic. It is a member of 1,3-thiazoles, a L-valine derivative, a carbamate ester, a member of ureas and a carboxamide.
Indications
Ritonavir is a prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of HIV infection in adults and children older than 1 month. Although Ritonavir is a potent inhibitor of HIV-1 and HIV-2 protease, it is not well tolerated in higher doses. It is mainly used in low doses to increase blood levels of other protease inhibitors and to extend their dosing interval. Ritonavir is more commonly associated with gastrointestinal side effects, altered taste sensation, paresthesias, and hypertriglyceridemia than are other protease inhibitors. Pancreatitis may occur in the presence or absence of hypertriglyceridemia.
brand name
Norvir (Abbott).
Antimicrobial activity
Ritonavir is active against HIV-1 and, to a lesser extent, HIV-2.
Acquired resistance
At antiretroviral doses resistance is associated with the presence of specific amino acid substitutions in the HIV protease at positions 82 and 84. Concern about the risk for selection of ritonavir resistance when used at a subtherapeutic ‘booster’ dose has so far not been borne out by clinical experience.
General Description
Ritonavir belongs to the group of protease inhibitors, which block the part of HIV called protease. Its mode of action involves binding to the protease active site and inhibiting the activity of the enzyme.
Biological Activity
Ritonavir is an HIV protease inhibitor. It inhibits recombinant HIV-1 protease by 79% when used at a concentration of 0.5 nM. It inhibits HIV-13B-induced cell death in MT-4 human T cell leukemia cells (EC50 = 25 nM) as well as cell death induced by HIV-1LAI, HIV-2ROD, and HIV-2EHO in human MT-2 cells (IC50s = 0.045, 0.13, and 0.24 μM, respectively). Ritonavir also inhibits the cytochrome P450 (CYP) isoform CYP3A (IC50 = 0.14 μM). It inhibits CYP-mediated oxidative metabolism of the HIV protease inhibitors saquinavir , indinavir , nelfinavir , and amprenavir in rat and human liver microsomes in a concentration-dependent manner. Ritonavir (10 mg/kg) also prevents decreases in plasma levels of these four compounds in rats. Formulations containing ritonavir have been used in the treatment of HIV-1 infection.
Biochem/physiol Actions
Ritonavir is an HIV protease inhibitor now used frequently as a booster of other protease inhbitors. Ritonavir inhibits cytochrome P450-3A4 (CYP3A4), a liver enzyme that normally metabolizes protease inhibitors. It has also been investigated as a possible anti-cancer agent.
Mechanism of action
Because of the strong CYP450-inhibiting effects of ritonavir, the drug has found value when used in fixed-dosage combinations with other PIs to block their metabolism and act as a booster for these drugs (lopinavir/ritonavir and tipranavir/ritonavir). In these cases, ritonavir is used in a subtherapeutic dose but boosts the effectiveness of the coadministered drug. The utilization of ritonavir in a therapeutic dose for treating HIV infections appears to be decreasing, but its utilization as a booster drug is finding favor.
Pharmacokinetics
Oral absorption: Not known/available
Cmax 600 mg twice daily: c. 11.2 mg/L
Cmin 600 mg twice daily: c. 3.7 mg/L
Plasma half-life: c. 3–5 h
Volume of distribution: c. 0.3–0.6 L/kg
Plasma protein binding: c. 97%
Absorption and distribution
Fasting and high-fat meals had no appreciable effect on oral absorption. It penetrates poorly into the CNS. The semen:plasma ratio is <0.04. It is distributed into breast milk.
Metabolism and excretion
Four oxidized metabolites have been identified, the major of which retains antiretroviral activity. Around 11% of the dose is excreted in urine, 4% as unchanged drug. The remainder is found in feces. Metabolites are eliminated primarily via the feces.
No dose adjustment is recommended in mild to moderate hepatic impairment. It should not be given to patients with severe hepatic impairment, nor should it be given as a pharmacokinetic enhancer to patients with decompensated liver disease.
Side effects
Full (antiretroviral) doses are associated with nausea, vomiting, diarrhea and fatigue in >20% of subjects. The degree to which ritonavir at low dose is associated with specific adverse events is uncertain. In HIV-negative healthy volunteers given ‘booster’ doses of 100 mg every 12 h, the concentration of total cholesterol, low-density cholesterol and triglycerides all increased, and the concentration of high-density cholesterol concentration fell.
Drug interactions
Ritonavir-boosted nirmatrelvir has significant drug-drug interactions, primarily due to the ritonavir component of the combination. Boosting with ritonavir, which is a strong CYP3A inhibitor and a P-glycoprotein inhibitor, is required to increase the exposure of nirmatrelvir to a concentration that is effective against SARS-CoV-2. However, it may also increase concentrations of certain concomitant medications, thereby increasing the potential for serious and sometimes life-threatening drug toxicities. Additionally, ritonavir is an inhibitor, inducer, and substrate of various other drug-metabolizing enzymes and/or drug transporters.
Metabolism
Ritonavir is extensively metabolised in the liver mainly by
cytochrome P450 isoenzymes CYP3A4 and to a lesser
extent by CYP2D6. Five metabolites have been identified
and the major metabolite has antiviral activity, but
concentrations in plasma are low.
About 86% of a dose is eliminated through the faeces
(both as unchanged drug and as metabolites) and about
11% is excreted in the urine.
storage
-20°C
References
1) Lea and Faulds (2018)?Ritonavir;?Drugs?52?541 2) Koudriakova?et al.?(1998)?Metabolism of the Human Immunodeficiency Virus Protease Inhibitors Indinavir and Ritonavir by Human Intestinal Microsomes and Expressed Cytochrome P4503A4/3A5: Mechanism-Based Inactivation of Cytochrome P3503A by Ritonavir;?Drug Metab. Dispos.?26?552 3) Rock?et al.?(2014)?Characterization of Ritonavir-Mediated Inactivation of Cytochrome P450 3A4;?Mol. Pharmacol.?86?665
Ritonavir Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hangzhou Proserre Chemical Co.,Ltd. | +86-571-86088112 | info@proserre.com | China | 1077 | 58 |
| XI'AN TIANGUANGYUAN BIOTECH CO., LTD. | +86-029-86333380 18829239519 | sales06@tgybio.com | China | 961 | 58 |
| Hubei Harvest Chemical CO.,Ltd | +86-13129915771 +86-15623179893 | wendy@hb-harvestchem.com | China | 931 | 58 |
| Shenzhen Monkono Technology Co.,Ltd | +86-17063441314 | sansbiotech@outlook.com | China | 677 | 58 |
| Hebei Lingding Biotechnology Co., Ltd. | +86-18031140164 +86-19933155420 | erin@hbldbiotech.com | China | 878 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Henan Tengmao Chemical Technology Co. LTD | +8615238638457 | salesvip2@hntmhg.com | China | 415 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +undefined8613073685410 | sales@ichemie.com | China | 985 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
Related articles
- Mechanism of action of Ritonavir
- Ritonavir is a peptidomimetic inhibitor of HIV-1 and HIV-2 proteases. It is marketed by Abbott Laboratories under the brand na....
- Apr 1,2022
- Application of Ritonavir
- Ritonavir is an important active pharmaceutical ingredients[1]. A synthetic chemical available for oral use as soft capsules ....
- Nov 6,2019
View Lastest Price from Ritonavir manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-25 | RITONAVIR
155213-67-5
|
US $1.00 / g | 1g | 99.99% | 20t | Jinan Jianfeng Chemical Co., Ltd | |
 |
2024-04-23 | Ritonavir
155213-67-5
|
US $1.00 / g | 1g | 99% | 10 tons | ANHUI WITOP BIOTECH CO., LTD | |
 |
2024-04-23 | Ritonavir
155213-67-5
|
US $1100.00-25000.00 / kg | 1kg | 99% | 1000KG | Great Innovation (Shanghai) Pharma Co., Ltd., |





