Prasugrel
- CAS No.
- 150322-43-3
- Chemical Name:
- Prasugrel
- Synonyms
- Effient;Prasugrel base;Efient;CS-1997;Prasita;Ly640315;Ly 640315;Prasugrel;Ly-640315;prasugren
- CBNumber:
- CB01463424
- Molecular Formula:
- C20H20FNO3S
- Molecular Weight:
- 373.44
- MDL Number:
- MFCD09954140
- MOL File:
- 150322-43-3.mol
| Melting point | 120.0 to 124.0 °C |
|---|---|
| Boiling point | 493.5±45.0 °C(Predicted) |
| Density | 1.347 |
| storage temp. | 2-8°C |
| solubility | DMSO: >5mg/mL (warmed at 60 °C) |
| pka | 3.65±0.20(Predicted) |
| form | powder |
| color | white to beige |
| InChIKey | DTGLZDAWLRGWQN-UHFFFAOYSA-N |
| CAS DataBase Reference | 150322-43-3(CAS DataBase Reference) |
| FDA UNII | 34K66TBT99 |
| ATC code | B01AC22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Safety Statements | 24/25 |
| WGK Germany | 3 |
| HS Code | 29349990 |
Prasugrel price More Price(41)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML0331 | Prasugrel ≥98% (HPLC) | 150322-43-3 | 10mg | $114 | 2024-03-01 | Buy |
| Sigma-Aldrich | Y0002119 | Prasugrel for system suitability European Pharmacopoeia (EP) Reference Standard | 150322-43-3 | Y0002119 | $150 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML0331 | Prasugrel ≥98% (HPLC) | 150322-43-3 | 50mg | $265.8 | 2024-03-01 | Buy |
| TCI Chemical | P2040 | Prasugrel >98.0%(HPLC)(T) | 150322-43-3 | 200mg | $138 | 2024-03-01 | Buy |
| Cayman Chemical | 14278 | Prasugrel ≥98% | 150322-43-3 | 250 mg | $148 | 2024-03-01 | Buy |
Prasugrel Chemical Properties,Uses,Production
General Description
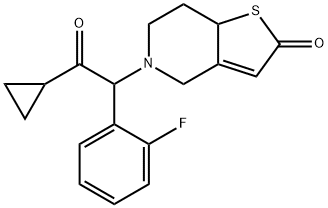
Prasugrel (brand name: “efficient”), a thienopyridine derivative, is a platelet activation and aggregation inhibitor structurally and pharmacologically related to clopidogrel and ticlopidine. Similar to clopidogrel, prasugrel is a prodrug that requires enzymatic transformation in the liver to its active metabolite, R-138727. R-138727 irreversibly binds to P2Y12 type ADP receptors on platelets thus preventing activation of the GPIIb/IIIa receptor complex. As a result, inhibition of ADP-mediated platelet activation and aggregation occurs. Prasugrel was developed by Daiichi Sankyo Co. and is currently marketed in the United States and Canada in cooperation with Eli Lilly and Company for acute coronary syndromes planned for percutaneous coronary intervention (PCI). FDA approved in 2009. Its brand name is “efficient”.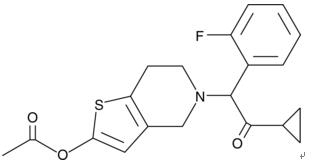
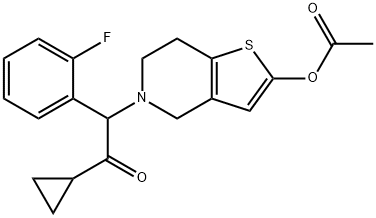
Figure 1 Molecular structure of Prasugrel
Chemical Properties
Prasugrel is usually formulated in the form of hydrochloride salt. Prasugrel hydrochloride has the empirical formula C20H20FNO3S•HCl representing a molecular weight of 409.90. Prasugrel hydrochloride is a white to practically white solid. It is soluble at pH 2, slightly soluble at pH 3 to 4, and practically insoluble at pH 6 to 7.5. It also dissolves freely in methanol and is slightly soluble in 1-and 2-propanol and acetone. It is practically insoluble in diethyl ether and ethyl acetate.
Application
Prasugrel is used in combination with low dose aspirin to prevent thrombosis in patients with acute coronary syndrome (ACS), including unstable angina pectoris, non-ST elevation myocardial infarction (NSTEMI), and ST elevation myocardial infarction (STEMI), who are planned for treatment with percutaneous coronary intervention (PCI). In studies, prasugrel was more effective than the related clopidogrel but also caused more bleeding. Overall mortality was the same.
Prasugrel does not change the risk of death when given to people who have had a STEMI or NSTEMI. Prasugrel does however increase the risk of bleeding and may decrease the risk of further cardiovascular problems. Thus routine use in NSTEMI patients is of questionable value.
Mode of action
Prasugrel is an inhibitor of platelet activation and aggregation through the irreversible binding of its active metabolite to the P2Y12 class of ADP receptors on platelets.
Pharmacodynamics
Prasugrel is a member of the thienopyridine class of ADP receptor inhibitors, like ticlopidine (trade name Ticlid) and clopidogrel (trade name Plavix). These agents reduce the aggregation ("clumping") of platelets by irreversibly binding to P2Y12 receptors. Compared to clopidogrel, prasugrel inhibits adenosine diphosphate–induced platelet aggregation more rapidly, more consistently, and to a greater extent than do standard and higher doses of clopidogrel in healthy volunteers and in patients with coronary artery disease, including those undergoing PCI. The increased potency of prasugrel appears to be due to more efficient conversion to its active metabolite. However, it carries a higher risk of bleed compared to clopidogrel, which may be a result of its higher potency. Prasugrel produces inhibition of platelet aggregation to 20 μM or 5 μM ADP, as measured by light transmission aggregometry.
Administration and dosage
Initiate Effient (the brand name of prasugrel) treatment as a single 60 mg oral loading dose and then continue at 10 mg orally once daily. Patients taking Effient should also take aspirin (75 mg to 325 mg) daily.
Metabolism
Prasugrel is not detected in plasma following oral administration. It is rapidly hydrolyzed in the intestine to thiolactone by human carboxylesterase (hCE) 2. This intermediate is further metabolized to its active metabolite, R-138727, in a single step by cytochrome P450 enzymes in the liver (primarily CYP3A4 and CYP2B6 and to a lesser extent by CYP2C9 and CYP2C19). The active metabolite is further metabolized by S-methylation or cysteine conjugation to two inactive metabolites. Unlike clopidogrel, transformation of prasugrel to its active metabolite does not appear to be affected by cytochrome P450 polymorphisms.
Adverse reaction
Common adverse reactions include Bleeding, thrombotic Thrombocytopenic Purpura and Hypersensitivity Including Angioedema.
In TRITON-TIMI 38, common and other important non-hemorrhagic adverse events were, for Effient and clopidogrel, respectively: severe thrombocytopenia (0.06%, 0.04%), anemia (2.2%, 2.0%), and abnormal hepatic function (0.22%, 0.27%), and allergic reactions (0.36%, 0.36%), and angioedema (0.06%, 0.04%).
Warning and precaution
CABG-related bleeding: Risk increases in patients receiving Effient who undergo CABG.
Discontinuation of Effient: Premature discontinuation increases risk of stent thrombosis, MI, and death.
Thrombotic thrombocytopenic purpura (TTP): TTP has been reported with Effient.
Contraindication
Prasugrel should not be given to patients with active pathological bleeding, such as peptic ulcer or a history of transient ischemic attack or stroke, because of higher risk of stroke (thrombotic stroke and intracranial hemorrhage).
Drug Interaction
Warfarin
Co-administration of Effient and warfarin increases the risk of bleeding.
Non-Steroidal Anti-Inflammatory Drugs
Co-administration of Effient and NSAIDs (used chronically) may increase the risk of bleeding.
Other Concomitant Medications
Effient can be administered with drugs that are inducers or inhibitors of cytochrome P450 enzymes. Effient can be administered with aspirin (75 mg to 325 mg per day), heparin, GPIIb/IIIa inhibitors, statins, digoxin, and drugs that elevate gastric pH, including proton pump inhibitors and H2 blockers.
Description
Prasugrel is a third-generation thienopyridine that has been developed and launched for the prevention of atherothrombotic events in patients with ACS or following PCI. While the second-generation agent clopidogrel was an improvement over the first-generation ticlopidine, which suffered from gastrointestinal adverse effects and the risk of neutropenia with prolonged use, its delayed onset of action and considerable interpatient variability prompted the search for the next-generation thienopyridine. The mechanism of action of these platelet inhibitors involves initial biological activation to a sulfhydryl metabolite that irreversibly binds to the P2γ12 receptor on platelets via disulfide formation, thereby preventing platelet activation and aggregation by the endogenous agonist adenosine diphosphate (ADP). The advantage of prasugrel over its predecessors is its more efficient and consistent absorption and rapid conversion to its active metabolite. Co-administration of thienopyridines with acetylsalicylic acid (aspirin), an inhibitor of the synthesis of the platelet aggregation mediator thromboxane A2, is an effective antiplatelet strategy and joins antagonists of glycoprotein IIb/IIIa, which target the final step in platelet aggregation, in the medical arsenal combating atherothrombotic events.
Originator
Daiichi Sankyo (Japan)
Uses
Inhibits platelet aggregation (platelet ADPP 2Y12 antagonist).
Uses
Prasugrel is a platelet inhibitor that reduces aggregation of platelets by being a P2Y12(ADP receptor) inhibitor.
Definition
ChEBI: 5-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl acetate is a member of the class of thienopyridines that is 2-acetoxy-4,5,6,7-tetrahydrothieno[3,2-c]pyridine in which the amino hydrogen is replaced by a 2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl group. It is an acetate ester, a member of cyclopropanes, a ketone, a member of monofluorobenzenes, a tertiary amino compound and a thienopyridine.
brand name
Effient
Clinical Use
Prasugrel is a platelet inhibitor developed by Daiichi Sankyo Co. and is marketed in the United States in cooperation with Eli Lilly and Company for acute coronary syndromes planned for percutaneous coronary intervention (PCI). Prasugrel was approved for use in Europe in February 2009, and is currently available in the UK. In the U.S. prasugrel is also approved for the reduction of thrombotic cardiovascular events, including stent thrombosis, in patients with acute coronary syndrome who are to be managed with PCI. Prasugrel is a member of the thienopyridine class of ADP receptor inhibitors, and irreversibly binds to P2Y12 receptors.
Side effects
In addition to the hemorrhagic side effect, other serious adverse events included AF, bradycardia, leucopenia, severe thrombocytopenia, angiodema, anemia, and abnormal hepatic function with hypertension, headache, back pain, dyspnea, nausea, dizziness, and diarrhea as less severe complaints. Prasugrel is contraindicated in patients with active pathological bleeding, such as peptic ulcers or intracranial hemorrhage, and in patients with a history of prior transient ischemic attack or stroke. In addition, in patients 75 years old, <60 kg, or likely to undergo urgent coronary artery bypass graft surgery, the risk may not outweigh the benefit. When possible, prasugrel treatment should be discontinued at least 7 days prior to any surgery. While warfarin and non-steroidal antiinflammatory drugs (NSAIDS) may increase the risk of bleeding with coadministration of prasugrel, no drug interactions are anticipated with concomitant use of drugs that are inducers or inhibitors of the cytochrome P450 enzymes. Prasugrel may also be administered with aspirin (75-325 mg per day), heparin, GP IIb/IIIa inhibitors, statins, digoxin, and drugs that elevate gastric pH, including PPIs and H2 blockers.
Synthesis
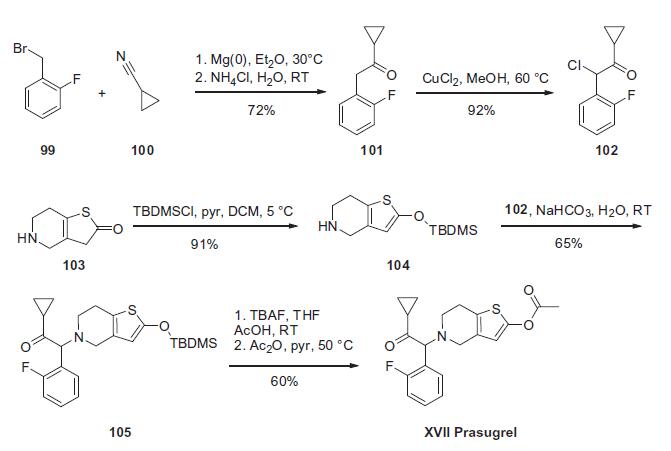
The synthesis of prasugrel begins with the preparation of the a-ketocyclopropane 102 which is prepared as summarized in the scheme. Conversion of 1-(bromomethyl)-2-fluorobenzene (99) to the corresponding Grignard reagent through reaction with magnesium followed by condensation with nitrile 100 resulted in ketone 101 in 72% yield. Chlorination of ketone 101 with CuCl2 resulted in the key prasugrel coupling component 102 in 92% yield. The piperidine coupling partner was prepared by treating thiolactone 103 with TBDMSCl and triethylamine to give thiophene 104 in 91% yield. Treatment of piperidine 104 with a-chloroketone 102 resulted in enol silane 105 in 65% yield. Reaction of silylenol ether 105 with acetic anhydride in the presence of triethylamine and catalytic DMAP resulted in the preparation of prasugrel (XVII) in 60% yield.
Drug interactions
Potentially hazardous interactions with other drugs
Anticoagulants: enhanced anticoagulant effect with
coumarins and phenindione.
Metabolism
Prasugrel is a prodrug and is rapidly metabolised in the liver by various cytochrome P450 enzymes to an active metabolite and inactive metabolites. The active metabolite is further metabolised to 2 inactive compounds which are excreted in the urine and faeces; about 68% of a dose is excreted in urine and about 27% in faeces.
storage
Store at +4°C
Prasugrel Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 1696 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Lianyungang happen teng technology co., LTD | 15950718863 | wang666xt@163.com | CHINA | 295 | 58 |
View Lastest Price from Prasugrel manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Prasugrel
150322-43-3
|
US $15.00 / kg | 1kg | 99.912% | 10ton | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-15 | Prasugrel
150322-43-3
|
US $1.00-100.00 / mg | 100mg | 99.0% | 5ton/month | ANHUI SHENGZHIKAI BIOTECHNOLOGY CO.,LTD | |
 |
2023-08-23 | Prasugrel
150322-43-3
|
US $30.00 / kg | 1kg | 99% | 1000t/year | Anhui Ruihan Technology Co., Ltd |





