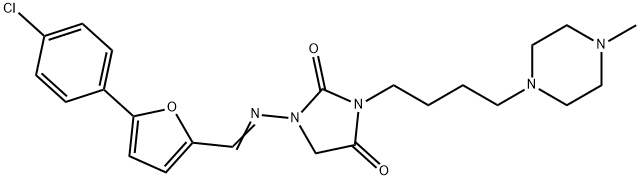
AZIMILIDE
- CAS No.
- 149908-53-2
- Chemical Name:
- AZIMILIDE
- Synonyms
- NE-10064;AZIMILIDE;Unii-74qu6p2934;AZIMILIDE USP/EP/BP;Azimilide (NE-10064);1-(((5-(4-Chlorophenyl)furan-2-yl)methylene)amino)-3-(4-(4-methylpiperazin-1-yl)butyl)imidazolidine-2,4-dione;1-[[[5-(4-Chlorophenyl)-2-furanyl]methylene]amino]-3-[4-(4-methyl-1-piperazinyl)butyl]-2,4-imidazolidinedione;2,4-Imidazolidinedione, 1-(((5-(4-chlorophenyl)-2-furanyl)methylene)amino)-3-(4-(4-methyl-1-piperazinyl)butyl)-
- CBNumber:
- CB0197878
- Molecular Formula:
- C23H28ClN5O3
- Molecular Weight:
- 457.95
- MDL Number:
- MFCD00867099
- MOL File:
- 149908-53-2.mol
- MSDS File:
- SDS
| Boiling point | 594.9±60.0 °C(Predicted) |
|---|---|
| Density | 1.32 |
| storage temp. | 2-8°C |
| solubility | <4.58mg/mL in DMSO |
| form | Powder |
| pka | 7.72±0.10(Predicted) |
| FDA UNII | 74QU6P2934 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P280-P305+P351+P338 |
AZIMILIDE price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Biorbyt Ltd | orb611846 | Azimilide | 149908-53-2 | 10mg | $248.2 | 2021-12-16 | Buy |
| Biorbyt Ltd | orb611846 | Azimilide | 149908-53-2 | 50mg | $544 | 2021-12-16 | Buy |
| Biorbyt Ltd | orb611846 | Azimilide | 149908-53-2 | 100mg | $912.9 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0009168 | AZIMILIDE 95.00% | 149908-53-2 | 100MG | $1091.48 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0009168 | AZIMILIDE 95.00% | 149908-53-2 | 1G | $2355.15 | 2021-12-16 | Buy |
AZIMILIDE Chemical Properties,Uses,Production
Originator
Stedicor,Procter and Gamble
Uses
Cardiac depressant (anti-arrhythmic).
Definition
ChEBI: Azimilide is an imidazolidine-2,4-dione.
Manufacturing Process
1-Phenylmethylenamino-3-(4-chlorobutyl)-2,4-imidazolidinedione
dihydrochloride is prepared by adding 60% sodium hydride in mineral oil (7.8
g, 0.1944 mole) over 1 h to a stirred solution o f 1-
(benzy1ideneamino)hydantoin [prepared as described by J. Gut, A Novacet,
and P. Fiedler, Coll. Czech. Chern. Comrnun., Vol. 33, pp. 2087-2096, No. 71,
1968] (39.5 g , 0.1944 mole) in dimethyl formamide (1000 ml). After
complete addition, the solution is heated at steam bath temperature
(approximately 100°C) for 1.5 h. The resulting mixture is allowed to cool to
room temperature (30°C).
While stirring at room temperature, 1-bromo-4-chlorobutane (100 g ,0.5832
mole, 3 eq.) is added in one portion. The mixture becomes exothermic
reaching around 35°C in approximately 30 min. The near-solution is heated a
t approximately 80°C by steam bath for 4 h, cooled and stirred for
approximately 8 h at room temperature (30°C). The cloudy mixture is filtered,
removing a small amount of insoluble solid. The filtrate is concentrated under
reduced pressure to a semi-solid residue. This residue is triturated with H2O
(400 ml), collected, recrystallized from acetonitrile, and then air-dried to give
43.1 g (0.1467 mole) of 1-phenylmethyleneamino-3-(4-chlorobutyl)-2,4-
imidazolidinedione.
A mixture of 1-phenylmethyleneamino-3-(4-chlorobutyl)-2,4-
imidazolidinedione (43.1 g, 0.1467 mole), acetone (1200 ml) and sodium
iodide (48.4 9, 0.3227 mole) is heated to reflux. Reflux is maintained for 5 h.
The mixture is filtered, collecting the insoluble. The filtrate is recharged with
sodium iodide (10.0 g) and reflux is resumed and is maintained for 15 h. After
cooling to room temperature, the mixture is filtered, removing the insoluble
NaCl (total recovery, 9.5 g, 110%). The filtrate is poured into H2O (2000 ml)
while stirring. After stirring for 30 min, the solid is collected and air-dried to
yield 51.5 g (0.1337 mole) of 1-phenylmethyleneamino-3-(4-iodobutyl)-2,4-
imidazolidinedione.
A solution of 1-phenylmethyleneamino-3-(4-iodobutyl)-2,4-imidazolidinedione
(10.0 g, 0.0260 mole), dimethylformamide (150.0 mg) and 1-
methylpiperazine (11.5 ml, 10.4 9, 0.1040 mole) is heated to reflux. Reflux is
maintained for 3 h. After cooling to approximately 40°C, the solution is
concentrated under reduced pressure by rotary evaporator to an oily-solid
residue. This residue is dissolved in H2O (200 ml) then made basic with
saturated NaHCO3 (200 ml). The resulting mixture is stirred for 2 h. The solid
is collected and air-dried, and is next dissolved in absolute ethanol (150 ml),
next filtered, then made acidic to wet litmus with EtOH/HCl. After cooling
several hours, the solid is collected and air-dried to give 8.04 g (0.0187 mole)
of 1-phenylmethyleneamino-3-[4-(4-methyl-1-piperazinyl)butyl]-2,4-
imidazolidinedione dihydrochloride.
A mixture of 1-phenylmethyleneamino-3-[4-(4-methyl-1-piperazinyl)butyl]-
2,4-imidazolidinedione dihydrochloride (8.04 g, 0.0187 mole), 2 N HCl (125
ml) and 5% Pd/C: 50% H2O (1.5 g) is subjected to hydrogen on a Parr
apparatus at 40 psi at room temperature. After 2 h, the catalyst is removed
by filtration. The filtrate is divided into two equal portions. Each is
concentrated under reduced pressure on a rotary 10 evaporator to an oily
residue of 1-amino-3-[4-(4-methyl-1-piperazinyl)butyl]-2,4-imidazolidinedione
hydrochloride.
A solution of 1-amino-3-[4-(4-methyl-1-piperazinyl)butyl]-2,4-
imidazolidinedione hydrochloride (0.0094 mole), dimethylformamide (75 ml)
and 5-(4-chlorophenyl)-2-furancarboxaldehyde [prepared as described in U.S.
Patent No. 20 4,882,354, to Huang et al., assigned to Norwich Eaton
Pharmaceuticals, Inc., issued November 21, 1984, see Cols. 7 and 8] (1.94 g
, 0.0094 mole) is stirred at room temperature for 72 h. The mixture is
filtered, collecting the solid. Recrystallization from absolute ethanol/H2O and
air-drying gives 2.63 g (0.0050 mole) of 1-[[[5-(4-chlorophenyl)-2-
furanyl]methylene]amino]3-[4-(4-methyl-1-piperazinyl)butyl]-2,4-
imidazolidinedion hydrochloride.
1-[[[5-(4-Chlorophenyl)-2-furanyl]methylene]amino]-3-[4-(4-methyl-1-
piperazinyl)butyl]-2,4-imidazolidinedione hydrochloride, (6.56 g, 0.0124 mole)
is dissolved in H2O (300 ml) and washed with (1x100 ml). The aqueous phase
is made basic with saturated NaHCO3 solution. The resulting mixture is
extracted with CH2Cl2 (4x100 ml). The extract is washed with saturated NaCl
(2x50 ml), dried over MgSO4 (activated charcoal), filtered and concentrated
under reduced pressure to a solid residue. This solid is triturated in anhydrous
ether, collected and air-dried. Recrystallization once from absolute EtOH and
then from toluene (activated charcoal), next washing with anhydrous ether
and air drying gives 2.05 g (0.0045 mole) of 1-[[[5-(4-chlorophenyl)-2-
furanyl]methylene]amino]-3-[4-(4-methyl-1-piperazinyl)butyl]-2,4-
imidazolidinedione.
Therapeutic Function
Antiarrhythmic
General Description
Azimilide, E-1-[[[5-(4-chlorophenyl)-2-furanyl]methylene]amino]-3-[4-(4-methyl-1-piperazinyl)butyl]-2,4-imidazolidinedione, is a class III agent that significantlyblocks the delayed rectifier potassium current,Iks, including the Ikr component. Its ability to block multichannelsmay be caused by a lack of the methane sulfonamidegroup that is common to other class III agents, whichselectively block the Ikr potassium current. It is believedthat blocking both Ikr and Iks potassium currents yields consistentclass III antiarrhythmic effects at any heart rate.
Clinical Use
Azimilde is another Class III antiarrhythmic agent structurally unrelated to any of the above agents. Azimilide is not available in the United States; it is only available in Europe. Following oral administration, the drug is completely absorbed, with no effect of food. Protein binding is 94%. It is metabolized in the liver to an active carboxylate metabolite, but its concentration in plasma is less than 5% of the parent compound. Thus, it is considered to be therapeutically inactive. Renal excretion is approximately 10%. Its elimination half-life is 3 to 4 days.
Enzyme inhibitor
This class ΙΙΙ antiarrhythmic drug and ion channel blocker (FW = 457.95 g/mol; CAS 149908-53-2), also known by the code name NE-10064 and systematic name, 1-({(E)-[5-(4-chlorophenyl)furan-2-yl]methylidene} amino)-3-[4-(4-methylpiperazin-1-yl)butyl]-imidazolidine-2,4-dione, blocks both the slow-activating IKs and rapidly activating IKr components of the delayed rectifier potassium current, distinguishing its action from conventional potassium channel blockers such as sotalol and dofetilide,which block only IKr. Azimilide prolongs the cardiac refractory period in a dose-dependent manner, as manifested by increases in action potential duration, QTc interval, and effective refractory period.
AZIMILIDE Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3791 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29474 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | gksales1@gk-bio.com | China | 9339 | 58 |
| Jilin Chinese Academy of Sciences-yanshen Technology | +undefined18143011203 | info@chemextension.com | China | 42057 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131981 | 58 |
| LGM Pharma | 1-(800)-881-8210 | inquiries@lgmpharma.com | United States | 2127 | 70 |
| Shanghai Famo Bio-chemical Technology Company Ltd. | +862136680027 15800370750 | sales@famobiotech.com | China | 500 | 56 |
View Lastest Price from AZIMILIDE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-08-12 | AZIMILIDE USP/EP/BP
149908-53-2
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited | |
 |
2021-07-20 | AziMilide
149908-53-2
|
US $1.00-1.00 / KG | 1g | 99% | 50tons | Shaanxi Dideu Medichem Co. Ltd | |
 |
2021-07-13 | Azimilide
149908-53-2
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- AZIMILIDE USP/EP/BP
149908-53-2
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- AziMilide
149908-53-2
- US $1.00-1.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd
-

- Azimilide
149908-53-2
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd