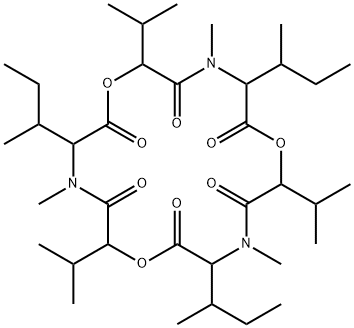
enniatin A
- CAS No.
- 2503-13-1
- Chemical Name:
- enniatin A
- Synonyms
- enniatin A;Lateritin I;N-Methylcyclo(L-Ile-D-Hmb-N-methyl-L-Ile-D-Hmb-N-methyl-L-Ile-D-Hmb-);(3S,6R,9S,12R,15S,18R)-3,9,15-tris[(2S)-butan-2-yl]-4,10,16-trimethyl-6,12,18-tri(propan-2-yl)-1,7,13-trioxa-4,10,16-triazacyclooctadecane-2,5,8,11,14,17-hexone;Cyclo[(2R)- 2-hydroxy-3-methylbutanoyl-N-methyl-L-isoleucyl-(2R)-2-hydroxy-3-methylbutanoyl-N-methyl-L-isoleucyl-(2R)-2-hydroxy-3-methylbutanoyl- N-methyl-L- isoleucyl]
- CBNumber:
- CB02079105
- Molecular Formula:
- C36H63N3O9
- Molecular Weight:
- 681.906
- MDL Number:
- MFCD19442801
- MOL File:
- 2503-13-1.mol
- MSDS File:
- SDS
| Melting point | 122-124 °C |
|---|---|
| Boiling point | 847.4±65.0 °C(Predicted) |
| Density | 1.022±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble10mg/mL |
| form | White to off-white crystalline solid. |
| pka | -0.96±0.70(Predicted) |
| FDA UNII | 59877B2B5T |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H301+H311+H331 |
| Precautionary statements | P261-P264-P280-P301+P310-P302+P352+P312-P304+P340+P311 |
| Hazard Codes | T |
| Risk Statements | 23/24/25 |
| Safety Statements | 45 |
| RIDADR | 2811 |
| WGK Germany | 3 |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| HS Code | 2934999090 |
enniatin A price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | E9661 | Enniatin A from Gnomonia errabunda, ≥95% (HPLC) | 2503-13-1 | 1mg | $219 | 2024-03-01 | Buy |
| Cayman Chemical | 17456 | Enniatin A ≥99% | 2503-13-1 | 500μg | $193 | 2024-03-01 | Buy |
| Cayman Chemical | 17456 | Enniatin A ≥99% | 2503-13-1 | 2.5mg | $816 | 2024-03-01 | Buy |
| TRC | E555990 | EnnlatinA | 2503-13-1 | 25mg | $11815 | 2021-12-16 | Buy |
| Usbiological | E3222-26 | Enniatin A | 2503-13-1 | 500ug | $426 | 2021-12-16 | Buy |
enniatin A Chemical Properties,Uses,Production
Description
Enniatins are cyclohexadepsipeptides commonly isolated from fungi that are known to have antibiotic properties and to induce apoptosis in several cancer lines. Many function as ionophores, forming pores in cellular membranes to allow selective ion transport. Enniatin A is one of four major analogs in the enniatin complex . It has been shown to moderately inhibit acyl-CoA:cholesterol acyltranferase activity in rat liver microsomes with an IC50 value of 22 μM. Enniatin A also demonstrates anthelmintic properties against N. brasiliensis, T. spiralis, and H. spumosa.
Uses
Enniatins are cyclohexadepsipeptides commonly isolated from fungi that are known to have antibiotic properties and to induce apoptosis in several cancer lines. Many function as ionophores, forming pores in cellular membranes to allow selective ion transport. Enniatin A is one of four major analogs in the enniatin complex . It has been shown to moderately inhibit acyl-CoA:cholesterol acyltranferase activity in rat liver microsomes with an IC50 value of 22 μM. Enniatin A also demonstrates anthelmintic properties against N. brasiliensis, T. spiralis, and H. spumosa.[Cayman Chemical]
Uses
Enniatins are a family of depsipeptide ionophores, produced by several Fusarium species. Recently, the effects of the enniatins on acyl-CoA cholesterol transferase, transporters and the selectivity of their antitumor action have received more focus. Enniatin A is one of four major analogues of the enniatin complex.
Uses
Enniatins are a family of depsipeptide ionophores, produced by several Fusarium species. More recently, the effects of the enniatins on acyl-CoA cholesterol transferase, transporters and the selectivity of their antitumour action have received more focus. Enniatin A is one of four major analogues of the enniatin complex and has not previously been available for investigation.
Definition
ChEBI: An enniatin obtained from formal cyclocondensation of three N-[(2R)-2-hydroxy-3-methylbutanoyl]-N-methyl-L-isoleucine units.
Biochem/physiol Actions
Enniatins are a group of cyclohexadepsipeptide mycotoxins produced by Gnomonia errabuda and several Fusaria species, with phytotoxic, antibiotic, and insecticidal activities. Enniatins function as ionophors by their incorporation into the cellular membrane to form dimeric structures that transport monovalent ions across the membrane (especially the mitochondrial membranes) affecting oxidative phosphorylation uncoupling. It has been demonstrated that enniatins have a cytotoxic effect on human cancer cells. Furthermore, incubation of H4IIE hepatoma cells with enniatins strongly diminished phosphorylation of the ERK (p44/p42).
enniatin A Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| BOC Sciences | +16314854226 | inquiry@bocsci.com | United States | 19743 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Sigma-Aldrich | 021-61415566 800-8193336 | orderCN@merckgroup.com | China | 51471 | 80 |
| Shanghai EFE Biological Technology Co., Ltd. | 021-65675885 18964387627 | info@efebio.com | China | 9709 | 58 |
| Lynnchem | 86-(0)29-85992781 17792393971 | info@lynnchem.com | China | 4587 | 58 |
| Novachemistry | 44-20819178-90 02081917890 | info@novachemistry.com | United Kingdom | 4381 | 58 |
| Shenzhen Regent Biochemical Technology Co., Ltd. | 0755-0755-85201366 18938635012 | sales@regentsciences.com | China | 9287 | 58 |
| Supplier | Advantage |
|---|---|
| ATK CHEMICAL COMPANY LIMITED | 60 |
| TargetMol Chemicals Inc. | 58 |
| Career Henan Chemica Co | 58 |
| BOC Sciences | 58 |
| Aladdin Scientific | 58 |
| Sigma-Aldrich | 80 |
| Shanghai EFE Biological Technology Co., Ltd. | 58 |
| Lynnchem | 58 |
| Novachemistry | 58 |
| Shenzhen Regent Biochemical Technology Co., Ltd. | 58 |