ZANAMIVIR HYDRATE
- CAS No.
- 139110-80-8
- Chemical Name:
- ZANAMIVIR HYDRATE
- Synonyms
- Zanamivir;Relenza;GANA;GG 167;Zanamir;Hsdb 7437;GR 121167X;139110-80-6;Unii-L6o3xi777i;GANA (inhibitor)
- CBNumber:
- CB0292498
- Molecular Formula:
- C12H20N4O7
- Molecular Weight:
- 332.31
- MDL Number:
- MFCD03791324
- MOL File:
- 139110-80-8.mol
- MSDS File:
- SDS
| Melting point | 2560C (dec.) |
|---|---|
| alpha | D20 +40.9° (c = 0.9 in water) |
| Density | 1.75±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble10mg/mL, clear |
| form | powder |
| pka | 3.82±0.70(Predicted) |
| color | white to beige |
| optical activity | [α]/D +30 to +40°, c = 1 in H2O |
| Water Solubility | Soluble to 5 mM in water |
| Stability | Hygroscopic |
| InChIKey | ARAIBEBZBOPLMB-UFGQHTETSA-N |
| CAS DataBase Reference | 139110-80-8 |
| NCI Dictionary of Cancer Terms | Relenza; zanamivir |
| FDA UNII | L6O3XI777I |
| NCI Drug Dictionary | Relenza |
| ATC code | J05AH01 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P301+P312+P330-P305+P351+P338 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-36/37/38 | |||||||||
| Safety Statements | 26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | RA9451000 | |||||||||
| HS Code | 2932999000 | |||||||||
| NFPA 704 |
|
ZANAMIVIR HYDRATE price More Price(46)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML0492 | Zanamivir ≥98% (HPLC) | 139110-80-8 | 10mg | $128 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1724088 | Zanamivir United States Pharmacopeia (USP) Reference Standard | 139110-80-8 | 200mg | $598 | 2024-03-01 | Buy |
| Cayman Chemical | 15123 | Zanamivir ≥98% | 139110-80-8 | 1mg | $33 | 2024-03-01 | Buy |
| Cayman Chemical | 15123 | Zanamivir ≥98% | 139110-80-8 | 5mg | $59 | 2024-03-01 | Buy |
| Sigma-Aldrich | Y0001708 | Zanamivir for system suitability European Pharmacopoeia (EP) Reference Standard | 139110-80-8 | y0001708 | $223 | 2024-03-01 | Buy |
ZANAMIVIR HYDRATE Chemical Properties,Uses,Production
Description
Zanamivir was launched as Relenza in Australia for treatment of human influenza A and B virus infections. Zanamivir (4-guanidino-Neu5Ac2en) can be obtained by several similar ways, for instance in seven step synthesis starting from N-acetyI-D-neuraminic acid. Mechanistically, Zanamivir is a potent and specific inhibitor of neuraminidase (or sialidase), a key viral surface glycohydrolase essential for viral replication and disease progression by catalyzing the cleavage of terminal sialic acid residues from the glycoprotein. The in vitro activity of Zanamivir against a wide variety of influenza A and B strains was demonstrated in different model systems; its activity against clinically relevant isolates of influenza virus, with IC50 values ranging from 0.005 to 15 pM was superior to those of amantadine and rimantadine.
Chemical Properties
Colorless Crystalline Solid
Originator
Biota Scientific Management (Australia)
Uses
Influenza viral neuraminidase inhibitor; structural analog of the sialic acid. Antiviral
Uses
antineoplastic, anti-metabolite
Uses
ZANAMIVIR HYDRATE is a sialic acid analog that inhibits neuraminidase release of newly replicated influenza virus particles. It has been shown to selectively inhibit the growth of influenza A and B viruses in plaque reduction assays with IC50 values ranging from 5 to 14 nM and to directly inhibit influenza A and B virus neuraminidases with IC50 values ranging from 0.6 to 7.9 nM in vitro. The efficacy and tolerabilbity of zanamivir has also been established in clinical trial.[Cayman Chemical]
Definition
ChEBI: Zanamivir is a member of guanidines. It has a role as an EC 3.2.1.18 (exo-alpha-sialidase) inhibitor and an antiviral agent.
Indications
Zanamivir (Relenza) is a neuraminidase inhibitor with activity against influenza A and B strains. Like oseltamivir, zanamivir is a reversible competitive antagonist of viral neuraminidase. It inhibits the release of progeny virus, causes viral aggregation at the cell surface, and impairs viral movement through respiratory secretions. Resistant variants with hemagglutinin and/or neuraminidase mutations have been produced in vivo; however, clinical resistance to zanamivir is quite rare at present.
Manufacturing Process
The 1st method of preparation of zanamivir:
5-(Acetylamino)-4-amino-2,6-anhydro-3,4,5-trideoxy-D-glycero-D-galacto-
non-2-enonic acid (3 g, 10.35 mmol) was suspended in methanol (37.5 ml)
and sodium acetate (1.89 g, 23.1 mmol) was added, causing a "caking" of the
suspension and making stirring difficult. To this at 21°C with exclusion of
moisture was added a solution of cyanogen bromide (1.14 g, 10.8 mmol) in
methanol (150 ml), in a dropwise manner. Stirring gradually became easier,
until a readily stirrable suspension was obtained. Addition was complete in 3.5
hours. The mixture was then stirred at 21°C with exclusion of moisture for 44
hours. The small amount of remaining solid was filtered off and solvent
evaporated in vacuo to an orange-brown foam. The foam was taken up in
methanol (125 ml) and with rapid stirring at 21°C was treated dropwise with
propan-2-ol (130 ml). The precipitate was filtered off, washed with iso-
PrOH/MeOH (3:2), and combined filtrate and washings evaporated to give the
5-(acetylamino)-4-cyanoamino-2,6-anhydro-3,4,5-trideoxy-D-glycero-D-
galacto-non-2-enonic acid as a pale yellow foam (3.48 g).
5-(Acetylamino)-4-cyanoamino-2,6-anhydro-3,4,5-trideoxy-D-glycero-D-
galacto-non-2-enonic acid (500 mg, 1.59 mmol) was dissolved in dried (over
3 A mol. sieves) methanol (20 ml) and anhydrous hydrazine (0.5 ml, 15.9
mmol) was added. This was then stirred at 21°C for 18 hours. The white
precipitate was filtered off, washed with methanol and air-dried (0.172 g,
31%). The solid was taken up in water (3.2 ml) and with warming and
swirling, propan-2-ol (8.1 ml) was added. The cystallised material was filtered
off, air-dried then dried under high vacuum to give D-glycero-D-galacto-non-
2-enonic acid, 5-(acetylamino)-4-(aminoiminomethyl)amino)-2,6-anhydro-
3,4,5-trideoxy as a white solid (0.127 g,); >97% purity; M.P. >180°C.
The 2nd method of preparation of zanamivir:
5-(Acetylamino)-4-cyanoamino-2,6-anhydro-3,4,5-trideoxy-D-glycero-D-
galacto-non-2-enopyranosonic acid (500 mg, 1.585 mmol) was dissolved in
dried (over 3 A mol. sieves) methanol (12 ml) and methylamine (33 wt. %
solution in ethanol, 1.93 ml, 15.85 mmol) was added. This was stirred at
21°C for 18 hours. The precipitate was filtered off and air dried to a white
solid (127 mg, 23%). This was recrystalIised from water (1.4 ml) and propan-
2-ol (6.9 ml). The product was filtered off and dried under high vacuum to
give the title compound as a white solid (56 mg, 10.2%). Concentration of
mother liquors gave a further 21.3 mg (4%) of D-glycero-D-galacto-non-2-
enonic acid, 5-(acetylamino)-4-(aminoiminomethyl)amino)-2,6-anhydro-3,4,5-
trideoxy-; 97.7% purity; M.P. >180°C.
brand name
Relenza (GlaxoSmithKline).
Therapeutic Function
Antiviral
Acquired resistance
Resistance is presently uncommon, including strains resistant to oseltamivir. In clinical trials the frequency was no more than 1% of exposed patients.
General Description
Zanamivir is identical to 2-deoxy-2,3-dehydro-N-acetylneuraminic acid except that itpossesses a guanidino group at position 4 instead of a hydroxylgroup. At positions 119 and 227 of the receptor site,there exist glutamic acid residues. Zanamivir has beenshown to form a salt bridge with the guanidine and Glu-119and a charge transfer interaction with Glu-227. These interactionsincrease the interaction strength with the enzymeand create an excellent competitive inhibitor and an effectiveantiviral agent for influenza types A and B.
Human studies have shown that zanamivir is effectivewhen administered before or after exposure to the influenzavirus. If administered before exposure to the virus, the drugreduced viral propagation, infectivity, and disease symptoms.If administered after exposure, the drug reduces propagation,viral titer, and illness. Zanamivir is marketed as a dry powderfor oral inhalation. It is used in adolescents and adults who have been exposed and are symptomatic for not more than 2days. Zanamivir is also indicated for prophylactically treatingfamily members of a person who has developed influenza.
Pharmaceutical Applications
A synthetic neuraminidase inhibitor formulated for administration by inhalation.
Biochem/physiol Actions
Zanamivir is an influenza viral neuraminidase inhibitor.
Pharmacology
Zanamivir is generally well tolerated. Bronchospasm and impaired lung function have been reported in some patients taking this medication, but many of these individuals had serious underlying pulmonary disease. Zanamivir should be discontinued if an individual develops bronchospasm or breathing difficulties; treatment and hospitalization may be required. Allergic reactions, including angioedema, have been rarely reported. The efficacy of zanamivir depends upon the proper use of the inhaler device.
Pharmacokinetics
Oral bioavailability is poor. After inhalation local respiratory mucosal concentrations greatly exceed those that are inhibitory for influenza A and B replication. The median concentrations in the sputum exceed 1 mg/L 6 h after inhalation and remain detectable for 24 h.
Clinical Use
Treatment of influenza A and B infections in patients over 7 years of age, and prophylaxis of patients ??5 years of age
Clinical Use
Zanamivir is indicated for treatment of uncomplicated acute influenza A and B virus in patients aged 7 and older.Treatment should be initiated no later than 2 days after the onset of symptoms. Zanamivir shortens the duration of illness by 1 to 1.5 days. It is also an effective prophylaxis against influenza; however, the FDA has not approved this indication at the time of publication.
Side effects
Zanamivir is contraindicated in individuals with severe or decompensated chronic obstructive lung disease or asthma because it has not been shown to be effective in these individuals and can cause serious adverse pulmonary reactions. Individuals with mild to moderate asthma may have a decline in lung function when taking zanamivir. The safety and efficacy of this medication have not been determined in individuals with severe renal insufficiency. No clinically significant drug interactions have been reported. Zanamivir does not decrease the effectiveness of the influenza vaccine.
Side effects
Most adverse effects are related to the respiratory tree. These include rhinorrhea and, rarely, bronchospasm. Nausea and vomiting have been reported at low incidence.
Metabolism
Zanamirvir is effective when administered via the nasal, intraperitoneal, and intravenous (IV) routes, but it is inactive when given orally. Animal studies have shown 68% recovery of the drug in the urine following intraperitoneal administration, 43% urinary recovery following nasal administration, and only 3% urinary recovery following oral administration. Human data gave results similar to those obtained in animal models. Human efficacy studies with nasal drops or sprays demonstrated that the drug was effective when administered before and after exposure to influenza A or B virus. When given before viral inoculation, the drug reduced viral shedding, infection, and symptoms. When administered beginning at either 26 or 32 hours after inoculation, there was a reduction in shedding, viral titer, and fever.
storage
Store at -20°C
References
[1] elliott m. zanamivir: from drug design to the clinic. philos trans r soc lond b biol sci, 2001, 356(1416): 1885-93.
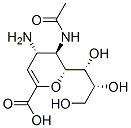
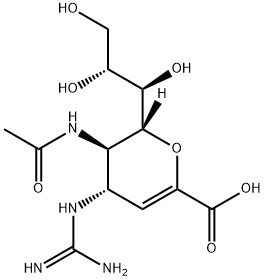
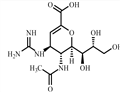
ZANAMIVIR HYDRATE Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Zhejiang ZETian Fine Chemicals Co. LTD | 18957127338 | stella@zetchem.com | China | 2141 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 967 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from ZANAMIVIR HYDRATE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-10 | Zanamivir
139110-80-8
|
US $0.00-0.00 / mg | 10mg | 90%+ | 10g | Guangzhou PI PI BIOTECH INC | |
 |
2023-03-22 | ZANAMIVIR HYDRATE
139110-80-8
|
US $100.00 / kg | 1kg | 99% | 20 tons | Hebei Duling International Trade Co. LTD | |
 |
2022-02-23 | ZANAMIVIR HYDRATE
139110-80-8
|
US $9.90-8.80 / g/Bag | 10g/Bag | 99.99%HPLC.USP42——Powder、Oil、Pills、Capsules、Tablets,Customiz | 1000kg/Month | Shanghai Biolang Biotechnology Co., Ltd. |
-

- Zanamivir
139110-80-8
- US $0.00-0.00 / mg
- 90%+
- Guangzhou PI PI BIOTECH INC
-

- ZANAMIVIR HYDRATE
139110-80-8
- US $100.00 / kg
- 99%
- Hebei Duling International Trade Co. LTD
-

- ZANAMIVIR HYDRATE
139110-80-8
- US $9.90-8.80 / g/Bag
- 99.99%HPLC.USP42——Powder、Oil、Pills、Capsules、Tablets,Customiz
- Shanghai Biolang Biotechnology Co., Ltd.
139110-80-8(ZANAMIVIR HYDRATE)Related Search:
1of4





