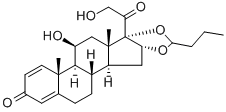
Budesonide
- CAS No.
- 51333-22-3
- Chemical Name:
- Budesonide
- Synonyms
- PULMICORT;Budeson;Respules;RHINOCORT;Budenofalk;Budesonide CRS;BIDIEN;S-1320;Entocort;Micronyl
- CBNumber:
- CB0313122
- Molecular Formula:
- C25H34O6
- Molecular Weight:
- 430.53
- MDL Number:
- MFCD00083259
- MOL File:
- 51333-22-3.mol
| Melting point | 221-232°C (dec.) |
|---|---|
| Boiling point | 464.79°C (rough estimate) |
| alpha | D25 +98.9° (c = 0.28 in methylene chloride) |
| Density | 1.1046 (rough estimate) |
| refractive index | 1.4593 (estimate) |
| storage temp. | Store at RT |
| solubility | Practically insoluble in water, freely soluble in methylene chloride, sparingly soluble in ethanol (96 per cent). |
| form | powder |
| pka | 12.87±0.10(Predicted) |
| color | White to Off-White |
| Water Solubility | 21.53mg/L(temperature not stated) |
| Merck | 14,1468 |
| BCS Class | 2 |
| InChIKey | VOVIALXJUBGFJZ-KWVAZRHASA-N |
| CAS DataBase Reference | 51333-22-3(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | budesonide |
| FDA UNII | Q3OKS62Q6X |
| ATC code | A07EA06,D07AC09,R01AD05,R03BA02 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H361-H332-H317-H334-H312 | |||||||||
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P201-P202-P281-P308+P313-P405-P501-P261-P271-P304+P340-P312-P280-P302+P352-P312-P322-P363-P501-P261-P285-P304+P341-P342+P311-P501 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 40-36/37/38-20/21/22 | |||||||||
| Safety Statements | 22-36-26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TU3723000 | |||||||||
| HS Code | 29372900 | |||||||||
| Toxicity | LD50 oral in rat: > 3200mg/kg | |||||||||
| NFPA 704 |
|
Budesonide price More Price(36)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | B7777 | Budesonide ≥99% | 51333-22-3 | 250mg | $260 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1078201 | Budesonide United States Pharmacopeia (USP) Reference Standard | 51333-22-3 | 200mg | $436 | 2024-03-01 | Buy |
| Cayman Chemical | 15407 | Budesonide ≥95% | 51333-22-3 | 50mg | $44 | 2024-03-01 | Buy |
| Cayman Chemical | 15407 | Budesonide ≥95% | 51333-22-3 | 100mg | $66 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR1178 | Budesonide Pharmaceutical Secondary Standard; Certified Reference Material | 51333-22-3 | 500mg | $126 | 2024-03-01 | Buy |
Budesonide Chemical Properties,Uses,Production
Description
Budesonide (brand name: Pulmicort) is a synthetic corticosteroid medication. It is indicated for the maintenance and prophylactic treatment of asthma. It is also used for the long-term management of chronic obstructive pulmonary disease (COPD). It is also useful for the treatment of inflammatory bowel disease including Crohn’s disease, ulcerative colitis and microscopic colitis, and also for treating allergic rhinitis. It exhibits potent glucocorticoid activity and weak mineralocorticoid activity in vivo. It is effective in inhibiting the activities of multiple cell types and mediators participating in either allergic or non-allergic-mediated inflammation.
indications
Budesonide is a commonly used in the treatment of bronchial asthma clinical drug in China.it can be used for non-hormone-dependent or hormone-dependent asthma, and chronic asthmatic bronchitis. Or it is used for the treatment of seasonal allergic rhinitis and many years occurring allergic rhinitis and it is used for preventing polyps regeneration after the removal of nasal polyps . It can also be used to treat various skin diseases.
Budesonide is a halogen-free, topically applied , adrenal corticosteroid,it has significant anti-inflammatory, anti-allergic, anti-itching and anti-exudative effects. Its local effects is the same as beclomethasone dipropionate , it is mainly used for bronchial asthma which in the past bronchodilator or an antiallergic agent can not well control. This product can relieve bronchial spasm, pulmonary inhalation has anti-inflammatory effects, long-term use is well tolerated, it can reduce oral glucocorticoid dosage, without systemic effects of corticosteroids. It has the same effect on Children and adults with asthma as beclomethasone dipropionate , it has good effect, and it can be tolerated in long-term use ,for glucocorticoid-dependent or glucocorticoid-independent patients, mist inhaled can control asthma attacks and can effectively relapse prevention, inhaled treatment for 3 to 6 months, improvement in lung function is shown,the number of acute episodes is reduced , recoverability of plasma corticosterone concentration is gradually increased, and the daily dose of oral corticosteroids can be reduced by 40% to 50%. Thus,for glucocorticoid-dependent asthma patients, especially in patients with a larger amount of dose,the product can be used as an ideal alternative to thedrug for oral administration. Glucocorticoid effects of budesonide are strong, but mineralocorticoid effects are weak . Animal tests show that the drug’s effects on glucocorticoid receptor affinity are 200 times of cortisone, anti-inflammatory effects when it is applied topically are 1,000 times of cortisone , and subcutaneous and oral anti-inflammatory effects are only 40 and 25 times stronger than cortisone .
Precaution and Inhibition
Take Caution when it is used in tuberculosis, airway fungal infection or viral infection. Avoid pregnancy administration. Oral steroid-dependent patients, should gradually reduce oral steroid dose after 10d use , until it reaches a stable respiratory capacity minimum. In severe stress, the dose of oral steroids should be increased, such as severe infection, trauma, surgery. When sputum thickens and congests the airway , the short-term oral steroid should be supplemented . Fast Reduction of Oral hormone, can cause withdrawal symptoms, such as arthritis, rhinitis, eczema, muscle and joint pain,which should be paid attention to prevent it.
Budesonide can be used for mild asthma, when asthma symptoms are obvious,they should be added with a larger dose of water-soluble corticosteroids or bronchodilators and antihistamines. Aerosol has throat irritation on individual patient , Candida albicans infections may occur , rinse mouth and throat immediately after inhalation which can reduce irritation.
The above information is edited by the chemicalbook of Tian Ye.
References
http://www.rxlist.com/entocort-drug/indications-dosage.htm
https://en.wikipedia.org/wiki/Budesonide
https://www.drugbank.ca/drugs/DB01222
Description
Budesonide is composed of a 1:1 mixture of epimers of the 16,17-butylacetal, creating a chiral center. The 22R-epimer binds to the GR with higher affinity than does the 22S-epimer (Table 33.5). The butyl acetal chain provided the highest potency for the homologous acetal chains. Its rate of topical uptake into epithelial tissue is more than 100 times faster than that for hydrocortisone and dexamethasone. Approximately 85% of the IV administered dose of budesonide undergoes extensive first-pass hepatic metabolism by CYP3A4 to its primary metabolites, 6β-hydroxybudesonide and 16α-hydroxyprednisolone, which have approximately 1/100 the potency of budesonide. This is an important inactivation step in limiting budesonide's systemic effect on adrenal suppression.
Description
Budesonide is a glucocorticoid and an agonist of glucocorticoid receptors (EC50 = 45.7 pM in a transactivation assay). It is selective for glucocorticoid over mineralocorticoid receptors (EC50 = 7,620 pM). Budesonide inhibits LPS-induced TNF-α release from human peripheral blood mononuclear cells (PBMCs; IC50 = 0.96 nM). It reduces levels of IL-1β and eotaxin in the lungs and the number of eosinophils and neutrophils in bronchoalveolar lavage fluid (BALF) in a rat model of ovalbumin-induced airway inflammation when administered at a dose of 3 mg/kg. Intracolonic administration of budesonide decreases colon wet weight and colonic myeloperoxidase (MPO) activity in a rat model of oxazolone-induced colitis. Formulations containing budesonide have been used in the treatment of Crohn''s disease, ulcerative colitis, allergic rhinitis, and asthma.
Chemical Properties
White Solid
Originator
Budecort,AstraZeneca
Uses
The oral capsule is used for the treatment of mild to moderate active Crohn's disease. The oral tablet is used for induction of remission in patients with active, mild to moderate ulcerative colitis. The oral inhalation formulation is used for the treatme
Uses
laxative, antineoplastic
Uses
Budesonide is a glucocorticoid steroid that activates the glucorcorticoid receptor with an EC50 value of 12.4 nM. Like other glucocorticoids, budesonide reduces inflammation and has utility in inflammatory diseases, like asthma and inflammatory bowel disease. Also like other glucocorticoids, budesonide may be abused by athletes.[Cayman Chemical]
Uses
A non-halogenated glucocorticoid related to triamcinolone hexacetonide. Used as an antiinflammatory agent
Indications
Budesonide is a synthetic corticosteroid having a potent glucocorticoid and weak mineralocorticoid activity. In standard in vitro and animal models, budesonide has an approximately 200-fold higher affinity for the glucocorticoid receptor and a 1000-fold higher topical antiinflammatory potency than cortisol.
Definition
ChEBI: A glucocorticoid steroid having a highly oxygenated pregna-1,4-diene structure. It is used mainly in the treatment of asthma and non-infectious rhinitis and for treatment and prevention of nasal polyposis.
Manufacturing Process
50 grams of desonide (16α-hydroxyprednisolone-16,17-acetonide) and immediately thereafter 12.5 ml of butyraldehyde were added to 500 ml of 70% hydrofluoric acid solution at -5°C, and the mixture was stirred at 0°C one hour and then poured into 5 liters of demineralized water at 0°C. The precipitate was filtered, washed to neutrality with water and dried under vacuum to give 51 g of pure budesonide with an A/B epimer ratio of 9/91.
brand name
Entocort (AstraZeneca); Pulmicort (AstraZeneca); Rhinocort (AstraZeneca).
Therapeutic Function
Corticosteroid
General Description
Budesonide, 16α,17-[butylidenebis-(oxy)]-11β,21-dihydroxypregna-1,4-diene-3, 20-dione (Entocort), in oral capsules is used to treat Crohn disease. Theaffinity for the GC is approximately 200-fold greater than thatof hydrocortisone and 15-fold greater than that of prednisolone.Budesonide is a mixture of epimers, with the 22Rform having twice the affinity for the GR of the S epimer.This GC is metabolized by CYP3A4, and its levels can be increasedin the presence of potent CYP3A4 inhibitors.Budesonide is also used in an inhaled formulation for thetreatment of asthma.
General Description
Budesonide (Pulmicort Turbohaler,Rhinocort) is extensively metabolized in the liver, with 85%to 95% of the orally absorbed drug metabolized by the firstpasseffect. The major metabolites are 6β-hydroxybudesonideand 16α-hydroxyprednisolone, both with less than1% of the activity of the parent compound. Metabolism involvesthe CYP3A4 enzyme, so coadministration of budesonidewith a known CYP3A4 inhibitor should be monitoredcarefully.
Biological Activity
Synthetic anti-inflammatory glucocorticoid that displays chemopreventive activity. Prevents formation of lung adenomas and adenocarcinomas in mice following inhalation or oral administration. Reverses DNA hypomethylation and modulates expression of cancer related genes.
Biochem/physiol Actions
Budesonide is a second generation glucocorticoid with low systemic absorption. It is used as an anti-inflammatory agent in the treatment of asthma, rhinitis, and inflammatory bowel disease. It inhibits the expression of chemokine mRNA and production of eotaxin and RANTES protein in primary human bronchial epithelial cells. Budesonide is currently in clinical trials for the prevention of lung cancer. It shows inhibitory effects on benzo[a]pyrene-induced carcinogenesis of the lung in mice.
Mechanism of action
Budesonide is an acetal formed between the 16α,17α-dihydroxyl groups and butanal. It is a nonhalogenated glucocorticoid with a 16,17-acetal that decreases the mineralocorticoid activity. In receptor affinity studies, the R-epimer was twofold more active than the S-epimer. Because the C-21 hydroxy is free, budesonide is not a prodrug and is active as administered. Only 34% of the metered dose of inhaled budesonide reaches the lung.
Pharmacology
While budesonide is well absorbed from the GI tract, its oral bioavailability is low (about 10%), primarily because of extensive first-pass metabolism in the liver. Two major metabolites (16α-hydroxyprednisolone and 6β- hydroxybudesonide) are formed via the cytochrome P450 3A enzyme. In vitro studies on the binding of the two primary metabolites to the corticosteroid receptor indicate that their affinity for the receptor is less than 1% of that of the parent compound. It is hoped that use of this drug will avoid the long-term adverse reactions seen with systemically active corticosteroids.
Clinical Use
Recently, budesonide (Entecort EC) has been approved for the treatment of mildly to moderately active Crohn’s disease involving the ileum and/or ascending colon.
Veterinary Drugs and Treatments
While there are inhalational forms of the medication for treating asthma or allergic rhinitis, most veterinary interest involves its potential oral use to treat inflammatory intestinal diseases in small animals that are either refractory to, or intolerant of, systemic steroids. In humans, oral budesonide is indicated for Crohn’s disease.
Drug interactions
Potentially hazardous interactions with other drugs
Aldesleukin: avoid concomitant use.
Antibacterials: metabolism accelerated by rifamycins.
Anticoagulants: efficacy of coumarins and
phenindione may be altered.
Antiepileptics: metabolism accelerated by
carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone.
Antifungals: concentration of inhaled and oral
budesonide increased by itraconazole and ketoconazole.
Antivirals: concentration of inhaled, intranasal and
rectal budesonide increased by ritonavir.
Cobicistat: concentration possibly increased by
cobicistat - increased risk of adrenal suppression.
Grapefruit juice: concentration of oral budesonide
increased - avoid.
Vaccines: high dose corticosteroids can impair
immune response to vaccines - avoid with live
vaccines.
Metabolism
Budesonide was metabolized three- to sixfold more rapidly than triamcinolone acetonide. The pharmacokinetics of budesonide after inhalation, oral, and IV administration displayed a mean plasma half-life of 2.8 hours and a systemic bioavailability of approximately 10% after oral administration (Table 33.5) (101). Pulmonary bioavailability is less than 40% after inhalation (70–75% after correction for the amounts of budesonide deposited in the inhalation device and oral cavity). No oxidative metabolism was observed in the lung. When given by inhalation, 32% of the dose is excreted in the urine as metabolites, 15% in the feces, and 41% of the dose remained in the mouthpiece of the inhaler. Following intranasal administration, very little of intranasal budesonide is absorbed from the nasal mucosa. Much of the intranasal dose (~60%) was swallowed, however, and remained in the GI tract to be excreted unchanged in the feces, whereas that fraction of the intranasal dose that was absorbed was extensively metabolized.
storage
Store at RT
References
[1] ricardo zollner1*, eduardo abib junior1,2*, luciana fernandes duarte2, maurício wesley perroud2andantonioricardo amarante2. bioequivalency study for inhaled drugs: a pharmacodynamic approac. bioequivalence & bioavailability
Budesonide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| BIONNA MEDICINE CO.,LTD | 01056380788-8515; +8618518759099 | 790226113@qq.com | China | 52 | 58 |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | marketing1@neostarunited.com | China | 8349 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 | bruce@xrdchem.cn | CHINA | 566 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 967 | 58 |
Related articles
- Budesonide: Uses, Mechenism of action, and Side effects
- Budesonide, sold under the brand name Pulmicort, among others, is a corticosteroid medication.
- Mar 22,2024
View Lastest Price from Budesonide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | Budesonide
51333-22-3
|
US $0.00 / mg | 100mg | ≥98% HPLC | 100KG | Changsha Staherb Natural Ingredients Co., Ltd. | |
 |
2024-04-22 | Budesonide
51333-22-3
|
US $0.00 / Kg/Bag | 2Kg/Bag | 0.99 | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-04-03 | Budesonide
51333-22-3
|
US $0.00-0.00 / Kg | 1Kg | 99.9% | 20tons | airuikechemical co., ltd. |
-

- Budesonide
51333-22-3
- US $0.00 / mg
- ≥98% HPLC
- Changsha Staherb Natural Ingredients Co., Ltd.
-

- Budesonide
51333-22-3
- US $0.00 / Kg/Bag
- 0.99
- Sinoway Industrial co., ltd.
-

- Budesonide
51333-22-3
- US $0.00-0.00 / Kg
- 99.9%
- airuikechemical co., ltd.
51333-22-3(Budesonide)Related Search:
1of4