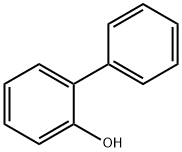
2-Phenylphenol
- CAS No.
- 90-43-7
- Chemical Name:
- 2-Phenylphenol
- Synonyms
- OPP;O-PHENYLPHENOL;Phenylphenol;O-HYDROXYBIPHENYL;BIPHENYL-2-OL;2-BIPHENYLOL;2-HYDROXYBIPHENYL;ORTHO-PHENYLPHENOL;o-Xenol;0-PHENYL PHENOL
- CBNumber:
- CB0365303
- Molecular Formula:
- C12H10O
- Molecular Weight:
- 170.21
- MDL Number:
- MFCD00002208
- MOL File:
- 90-43-7.mol
- MSDS File:
- SDS
| Melting point | 57-59 °C(lit.) |
|---|---|
| Boiling point | 282 °C(lit.) |
| Density | 1.21 |
| vapor pressure | 7 mm Hg ( 140 °C) |
| FEMA | 3959 | 2-PHENYLPHENOL |
| refractive index | 1.6188 (estimate) |
| Flash point | 255 °F |
| storage temp. | Store below +30°C. |
| solubility | Soluble in ethanol, acetone, benzene,sodium hydroxide, chloroform, acetonitrile, toluene, hexane, ligroin, ethyl ether, pyridine, ethylene glycol, isopropanol, glycol ethers and polyglycols. |
| form | Crystalline Flakes |
| pka | 10.01(at 25℃) |
| color | White |
| PH | 7 (0.1g/l, H2O, 20℃) |
| Odor | nearly wh. or lt. buff crystals, mild char. sweetish odor |
| explosive limit | 1.4-9.5%(V) |
| Water Solubility | 0.7 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,7304 |
| JECFA Number | 735 |
| BRN | 606907 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, halogens. |
| InChIKey | LLEMOWNGBBNAJR-UHFFFAOYSA-N |
| LogP | 3.18 at 22.5℃ |
| Substances Added to Food (formerly EAFUS) | O-PHENYLPHENOL |
| FDA 21 CFR | 175.105 |
| CAS DataBase Reference | 90-43-7(CAS DataBase Reference) |
| EWG's Food Scores | 6-9 |
| FDA UNII | D343Z75HT8 |
| Proposition 65 List | o-Phenylphenol |
| ATC code | D08AE06 |
| IARC | 3 (Vol. 73) 1999 |
| NIST Chemistry Reference | o-Hydroxybiphenyl(90-43-7) |
| EPA Substance Registry System | 2-Phenylphenol (90-43-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H318-H335-H410 | |||||||||
| Precautionary statements | P261-P264-P273-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,N | |||||||||
| Risk Statements | 36/37/38-50-37/38/50-36 | |||||||||
| Safety Statements | 22-61 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | DV5775000 | |||||||||
| Autoignition Temperature | >520 °C | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29071900 | |||||||||
| Toxicity | LD50 orally in rats: 2.48 g/kg (Hodge) | |||||||||
| NFPA 704 |
|
2-Phenylphenol price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | P28263 | 2-Phenylphenol 99% | 90-43-7 | 2kg | $250 | 2024-03-01 | Buy |
| Sigma-Aldrich | 45529 | 2-Phenylphenol PESTANAL , analytical standard | 90-43-7 | 250mg | $49.1 | 2024-03-01 | Buy |
| TCI Chemical | P0200 | 2-Phenylphenol >99.0%(GC) | 90-43-7 | 25g | $26 | 2024-03-01 | Buy |
| TCI Chemical | P0200 | 2-Phenylphenol >99.0%(GC) | 90-43-7 | 500g | $92 | 2024-03-01 | Buy |
| Alfa Aesar | A10592 | 2-Phenylphenol, 99% | 90-43-7 | 250g | $42.9 | 2024-03-01 | Buy |
2-Phenylphenol Chemical Properties,Uses,Production
Description
2-phenylphenol(OPP) is a broad spectrum fungicide used to protect crops in storage. It is highly soluble in water, moderately voatile but is not expected to be persistent in the environment. OPP is more selective than other free phenols but does produce phytotoxic effects.
Chemical Properties
o-Phenylphenol is a white to buff-colored crystalline solid with a distinct odor. When heated to decomposition, it emits acrid smoke and irritating fumes.
Uses
2-Phenylphenol is a agriculture fungicide and is no longer used as a food additive.
Production Methods
OPP is produced as a by-product in the manufacture of diphenyl oxide or by aldol condensation of hexazinone.
Definition
ChEBI: 2-Phenylphenol is a member of the class of hydroxybiphenyls that is biphenyl substituted by a hydroxy group at position 2. It is generally used as a post-harvest fungicide for citrus fruits. It has a role as an environmental food contaminant and an antifungal agrochemical. It derives from a hydride of a biphenyl.
Preparation
2-Phenylphenol can be recovered from the distillation residue of the process of phenol production via sulfonation. The phenol distillation residue contains about 40% of phenyl phenol with the other components including phenol, inorganic salts, water and so on. After vacuum distillation, the mixed phenyl phenol fraction is separated out with the vacuum being 53.3-66.7kPa. The temperature, started to be cut at 65-75 ℃ to until 100 ℃ above, but should not higher than 1345 ℃. Then take advantage of the solubility difference of ortho, p-hydroxy biphenyl in the trichlorethylene, the two are separated into pure product. The mixed material (mainly 2-hydroxy biphenyl and 4-hydroxy biphenyl) is heated to be dissolved in the trichlorethylene, after cooling, first precipitate out 4-hydroxy biphenyl crystal. After centrifuge filtration, dry to obtain 4-hydroxy biphenyl. The mother liquor was washed with a sodium carbonate solution, followed by dilute alkaline to make the 2-hydroxybiphenyl salt. After standing stratification, take the upper 2-hydroxybiphenyl sodium salt for dehydration under reduced pressure, namely, sodium salt products. The 2-hydroxybiphenylsodium salt is white to light red powder, being easily soluble in water with the solubility in 100g of water being 122g. The pH value of the 2% aqueous solution is 11.1-12.2. It is also easily soluble in acetone, methanol, soluble in glycerol, but insoluble in oil. The sodium salt of 2-hydroxy biphenyl, after acidification, can lead to the formation of 2-hydroxy biphenyl with both of them being food additives.
Application
2-phenylphenol is remarkably versatile organic chemical products, widely used antiseptic, auxiliaries and surfactant synthesis of new plastics, resins and polymer materials in areas such as stabilizers and flame retardants. It is used for the post-harvest control of storage diseases of apples, citrus fruit, stone fruit, tomatoes, cucumbers and other vegetables. It is also used for the protection of textiles and timber and as a fungistat in water-soluble paints.
Synthesis Reference(s)
Journal of the American Chemical Society, 75, p. 2947, 1953 DOI: 10.1021/ja01108a047
Tetrahedron, 40, p. 4981, 1984 DOI: 10.1016/S0040-4020(01)91336-5
The Journal of Organic Chemistry, 26, p. 283, 1961 DOI: 10.1021/jo01060a632
General Description
Light lavender crystals or solid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2-Phenylphenol react as a weak organic acid. Exothermically neutralizes bases. May react with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides to generate flammable gas (H2) and the heat of the reaction may ignite the gas. Is sulfonated very readily (for example, by concentrated sulfuric acid at room temperature) in exothermic reactions. May be nitrated very rapidly. Nitrated phenols often explode when heated and also form metal salts that tend toward detonation by rather mild shock. Can react with oxidizing agents .
Fire Hazard
2-Phenylphenol is combustible.
Flammability and Explosibility
Non flammable
Agricultural Uses
Fungicide, Disinfectant, Microbiocide: Used to make fungicides. Also used to make dye stuffs and rubber chemicals, but used primarily as a disinfectant cleaner. Registered for use in the U.S. and U.K.
Trade name
ANTHRAPOLE 73®; DOWCIDE-1®; Invalon OP®; KIWI LUSTR-277®; NECTRYL®; PREVENTOL-O Extra®; REMOL TRF®; TETROSIN OE®; TETROSIN OE-N®; TORSITE®; TUMESCAL OPE®
Safety Profile
A poison by intraperitoneal route.Moderately toxic by ingestion and possibly other routes.An experimental teratogen. Other experimentalreproductive effects. Human mutation data reported.Severe eye and moderate skin irritant. Questionablecarcinogen wi
Potential Exposure
o-Phenylphenol is used in the manufacture of plastics, resins, rubber, as Agricultural chemical, in making fungicides; as an intermediate in making dye stuffs and rubber chemicals; a germicide; used in food packaging.
Carcinogenicity
IARC classified SOPP as a B2
carcinogen in 1983, based on reports from Japan that high dietary levels of this sodium salt caused bladder tumors in
male rats. Both sodium saccharin and sodium
cyclamate also cause bladder tumors at high doses in male
rats, but classification of these food additives as B2 carcinogens
was recently rescinded by IARC at a meeting in 1998.
The U.S. National Toxicology Program conducted a skin
painting study with OPP in groups of 50 mice per sex. The
OPP was applied as an acetone solution on 3 days per week
for 2 years, both alone and as a promoter with DMBA.
No skin neoplasms were observed in either sex treated
with OPP alone, and there were no tumor enhancing or
inhibiting effects when OPP and DMBA were given in
combination.
Metabolic pathway
2-Phenylphenol is not used on growing plants because it is too phytotoxic
and there appears to be no information published on its metabolism in
plants. Its widespread use as a preservative, disinfectant and fungistat on
stored food (either by direct application or impregnated in packaging)
requires studies on its environmental fate and metabolism in mammals.
Several studies in mammals are available and the compound has been the
subject of an evaluation by the UK MAFF Pesticide Safety Directorate
(PSD); the results have been published (PSD, 1993). This evaluation was
prompted by the discovery of bladder tumours in rats treated with high
doses of the compound.
2-Phenylphenol is also used as the sodium and potassium salts where
water solublity is important. No information is available specifically on
the latter. The metabolism of the free phenol and the sodium salt have
been studied separately. Once absorbed into a cell, provided that internal
pH control is maintained, the two forms should be indistinguishable.
Shipping
UN3143 Dyes, solid, toxic, n.o.s. or Dye intermediates, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Purification Methods
Crystallise it from pet ether. [Beilstein 6 IV 4579.]
Toxicity evaluation
2-phenylphenol has a moderate to low toxicity to biodiversity. This substance has a low oral mammalian toxicity, is carcinogenic, a neurotoxin and recognised irritant. 2-Phenylphenol and its sodium salt have a low acute toxicity in mammals when administered orally, with LD50 values ranging from 600 to 3500 mg/kg of body weight.
http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/1340.htm
Degradation
2-Phenylphenol is readily degraded in surface waters and municipal waste mixtures, and the degradation is biologically mediated. In river water, radiolabelled 2- phenylphenol at concentrations ranging from 1.2 to 120 μg/litre was degraded to about 50% of the initial concentration in 1 week. The addition of mercuric chloride to inhibit biological activity reduced the decrease to only 10% after 30 days. In activated sludge, radiolabelled 2-phenylphenol at 9.6 mg/litre was degraded to 50% of the initial concentration in 24 h. 2-Phenylphenol therefore meets the criteria to be classified as readily biodegradable (FAO/WHO, 1999).
Incompatibilities
Strong bases, strong oxidizers
Waste Disposal
In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
2-Phenylphenol Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 | daisy@crovellbio.com | China | 5964 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7613 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Dalian Richfortune Chemicals Co., Ltd | 0411-84820922 8613904096939 | sales@richfortunechem.com | China | 303 | 57 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
Related articles
- The review of 2-phenylphenol
- 2-phenylphenol, also known as 2-hydroxybiphenyl or 2-phenylphenol, is a white or light yellow or light red powder, flakes or b....
- Feb 24,2022
View Lastest Price from 2-Phenylphenol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-12-23 | 2-Phenylphenol
90-43-7
|
US $50.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-09-06 | 2-Phenylphenol
90-43-7
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2023-08-25 | 2-Phenylphenol
90-43-7
|
US $15.00 / KG | 1KG | 99.5% | 1000tons | Hebei Guanlang Biotechnology Co,.LTD |
-

- 2-Phenylphenol
90-43-7
- US $50.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- 2-Phenylphenol
90-43-7
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- 2-Phenylphenol
90-43-7
- US $15.00 / KG
- 99.5%
- Hebei Guanlang Biotechnology Co,.LTD