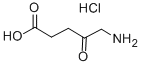
5-Aminolevulinic acid hydrochloride
- CAS No.
- 5451-09-2
- Chemical Name:
- 5-Aminolevulinic acid hydrochloride
- Synonyms
- 5-Ala;5-AMINOLEVULINIC ACID HCL;5-Aminolevulinic;inic acid hydrochL;5-AMINO-4-OXOPENTANOIC ACID HYDROCHLORIDE;evuL;Gliolan;5-AminoL;DELTA-AMINOLEVULINIC ACID HYDROCHLORIDE;ALA-PDT
- CBNumber:
- CB0496757
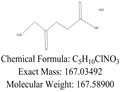
- Molecular Formula:
- C5H10ClNO3
- Molecular Weight:
- 167.59
- MDL Number:
- MFCD00012869
- MOL File:
- 5451-09-2.mol
| Melting point | ~150 °C (dec.) |
|---|---|
| Flash point | 155-157°C |
| storage temp. | -20°C |
| solubility | H2O: 50 mg/mL |
| form | powder |
| color | White to pale yellow |
| PH | pH (10g/l, 25℃) : 2.5~3.0 |
| Water Solubility | Soluble in dimethyl sulfoxide, methanol and water. |
| Decomposition | 155-157 ºC |
| Sensitive | Hygroscopic |
| Merck | 14,446 |
| BRN | 3690651 |
| Stability | Hygroscopic |
| InChIKey | ZLHFONARZHCSET-UHFFFAOYSA-N |
| CAS DataBase Reference | 5451-09-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | aminolevulinic acid hydrochloride; Levulan Kerastick |
| FDA UNII | V35KBM8JGR |
| NCI Drug Dictionary | aminolevulinic acid hydrochloride |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319 | |||||||||
| Precautionary statements | P264-P280-P302+P352+P332+P313+P362+P364-P305+P351+P338+P337+P313 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36/37/38-66-20/21/22-36/38 | |||||||||
| Safety Statements | 26-36/37-37/39-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OI1640000 | |||||||||
| F | 3-8-10 | |||||||||
| Hazard Note | Irritant | |||||||||
| HS Code | 29224999 | |||||||||
| NFPA 704 |
|
5-Aminolevulinic acid hydrochloride price More Price(60)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A7793 | 5-Aminolevulinic acid hydrochloride BioReagent, suitable for cell culture, powder, ≥98% | 5451-09-2 | 10MG | $30.1 | 2023-06-20 | Buy |
| Sigma-Aldrich | A7793 | 5-Aminolevulinic acid hydrochloride BioReagent, suitable for cell culture, powder, ≥98% | 5451-09-2 | 500MG | $175 | 2023-06-20 | Buy |
| Sigma-Aldrich | A7793 | 5-Aminolevulinic acid hydrochloride BioReagent, suitable for cell culture, powder, ≥98% | 5451-09-2 | 1G | $302 | 2023-06-20 | Buy |
| Sigma-Aldrich | A3785 | 5-Aminolevulinic acid hydrochloride ≥98% | 5451-09-2 | 500MG | $151 | 2023-06-20 | Buy |
| Sigma-Aldrich | A3785 | 5-Aminolevulinic acid hydrochloride ≥98% | 5451-09-2 | 1G | $272 | 2023-06-20 | Buy |
5-Aminolevulinic acid hydrochloride Chemical Properties,Uses,Production
Description
5-Aminolevulinic acid hydrochloride (5-ALA) is a naturally occurring amino acid that is an intermediate in the biosynthesis of chlorophyll and haemoglobin. It has anti-inflammatory, antioxidant and antitumour activities, and 5-Aminolevulinic acid hydrochloride-mediated sonodynamic therapy (SDT) has antitumour effects on pancreatic cancer cells. In addition, it is a visualising agent that causes high-grade gliomas to fluoresce under blue light, which can help guide surgeons in removing tumours and is considered a useful imaging tool for brain tumour resection.
Chemical Properties
white to pale yellow crystals or
Originator
Levulan Kerastick,DUSA Pharmaceuticals Inc.
Uses
5-Aminolevulinic acid hydrochloride finds an important role as a precursor in the synthesis of tetrapyrroles such as chlorophyll and heme. It is widely utilized in photodynamic therapy of diseases namely, Paget?s disease and human papillomavirus (HPV) infection-associated cervical condylomata acuminata.
Uses
Naturally occurring amino acid; precursor of tetrapyrroles in the biosynthesis of chlorophyll and heme. Antineoplastic (photosensitizer).
Uses
5-Aminolevulinic acid hydrochloride has been used as a supplement for culturing Escherichia coli cells for heme biosynthesis.
Application
5-Aminolevulinic acid hydrochloride has been used to activate or inhibit heme biosynthesis in HeLa and K562 cell lines. It has also been used in photodynamic therapy in mice with A431 tumours. Clinical oral administration of 5-Aminolevulinic acid hydrochloride potentiates the antihypertensive effects associated with anaesthetics and is used in the treatment of hypertensive patients, particularly in elderly hypertensive patients receiving antihypertensive medications[1-2].
Definition
ChEBI: A hydrochloride that is the monohydrochloride of 5-aminolevulinic acid. It is metabolised to protoporphyrin IX, a photoactive compound which accumulates in the skin. Used in combination with blue light illumination for the treatment of minimally to moderat ly thick actinic keratosis of the face or scalp.
Manufacturing Process
1) Oxidation Step
2.27 g (10.0 mmol) of N-furfurylphthalimide was charged into a three-necked
glass flask equipped with an oxygen feed tube, a thermometer, and a reflux
condenser, and dissolved in 100 ml of anhydrous pyridine. After the addition
of 7.0 mg of Rose Bengal, oxygen gas was fed at a rate of 20 ml/min at 10°-
20°C under irradiation by light. A 27 W white fluorescent lamp was used as a
light source and the radiation was performed from the outside of the flask.
After 7 hours, the irradiation was terminated and the pyridine was evaporated
under reduced pressure to obtain 2.47 g of a light brown, semi-crystalline
product.
2) Reduction Step (Hydrogenation)
2.00 g of the semi-crystalline solid obtained in (1) was dissolved in 40 ml of
methanol and stirred at 50°C in a hydrogen atmosphere under atmospheric
pressure in the presence of 200 mg of 5% palladium-on-carbon catalyst.
After five hours, the reaction was terminated and the mixture was allowed to
cool to room temperature. The catalyst was removed by filtration and
methanol was evaporated to obtain 2.11 g of white crystals.
The crystals were identified to be 5-phthalimidolevulinic acid by NMR analysis.
The yield was 97%.
3) Hydrolysis Step
100 ml of 6 N hydrochloric acid was added to 2.11 g of the white crystals (2),
and the mixture was heated under reflux for 5 hours.
After evaporating the hydrochloric acid under reduced pressure, a brown solid
product was obtained and dissolved in ethanol. Acetone was added to the
solution and the crystals produced were collected by filtration to obtain 0.689
g of 5-aminolevulinic acid hydrochloride. The yield based on Nfurfurylphthalimide
was 51%.
NMR spectrum data conformed to 5-aminolevulinic acid hydrochloride
Therapeutic Function
Photosensitizer
Biochem/physiol Actions
5-Aminolevulinic acid (5-ALA) is an intermediate in heme biosynthesis and is useful in cancer treatment. It is a non-protein amino acid. 5-ALA also has applications in the field of agriculture. It is being studied as an inducing reagent for protoporphyrin IX (PPIX) dependent fluorescence diagnosis of metastatic lymph nodes. 5-ALA is used for photodynamic therapy of diseases, such as Paget′s disease and HPV infection-associated cervical condylomata acuminata.
Purification Methods
Dry ALA-HCl in a vacuum desiccator over P2O5 overnight, then crystallise it by dissolving it in cold EtOH and adding dry Et2O. Also crystallis
References
[1] Oral Aminolevulinic Acid Hydrochloride[J]. Definitions, 2020. DOI:10.32388/vh56ni.
[2] NOBORU FUKUDA . 5-Aminolevulinic acid hydrochloride enhances bupivacaine-induced hypotension in spontaneously hypertensive rats[J]. Journal of pharmacological sciences, 2023. DOI:10.1016/j.jphs.2023.02.007.
5-Aminolevulinic acid hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Pharmyao, All for life--- A Specific Trading Platform For Reference Standard Material | +86-020-81716320 +8613602409664 | sales@pharmyao.com | China | 173 | 58 |
| EAST STAR BIOTECH (SUZHOU) CO., LTD. | +8617706846989 | marketing@eaststarbio.com | China | 48 | 58 |
| ZhenYiBio Technology Inc | +8615309206328 | alexxue@zhenyibio.com | China | 299 | 58 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| Jiangsu shring Biopharma Co., Ltd. | +8613372282299 | sales@shringchem.com | China | 3545 | 58 |
| BINBO BIOLOGICAL CO.,LTD | +8618629063126 | info@binbobiological.com | China | 268 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | kyra@quanjinci.com | China | 1532 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
Related articles
- Property, Synthesis and Clinical Effect of 5-aminolevulinic acid hydrochloride
- The physiological substance and precursor of the heme synthesis 5-aminolevulinic acid (ALA) is a promising prodrug for photodi....
- Jul 12,2022
View Lastest Price from 5-Aminolevulinic acid hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | 5-Aminolevulinic acid hydrochloride
5451-09-2
|
US $200.00-170.00 / KG | 1KG | 99% | 20 tons | Hong Kong Excellence Biotechnology Co., Ltd. | |
 |
2024-04-22 | 5-ALA
5451-09-2
|
US $0.00 / kg | 1kg | 99.8% | 1000 kg | BINBO BIOLOGICAL CO.,LTD | |
 |
2024-04-10 | 5-Aminolevulinic Acid Hydrochloride | US $0.00-0.00 / mg | 10mg | 90%+ | 10g | Guangzhou PI PI BIOTECH INC |
-

- 5-Aminolevulinic acid hydrochloride
5451-09-2
- US $200.00-170.00 / KG
- 99%
- Hong Kong Excellence Biotechnology Co., Ltd.
-

- 5-ALA
5451-09-2
- US $0.00 / kg
- 99.8%
- BINBO BIOLOGICAL CO.,LTD
-

- 5-Aminolevulinic Acid Hydrochloride
- US $0.00-0.00 / mg
- 90%+
- Guangzhou PI PI BIOTECH INC