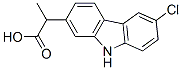
Carprofen
- CAS No.
- 53716-49-7
- Chemical Name:
- Carprofen
- Synonyms
- c5720;imadyl;rimadyl;Ridamyl;Caroline;CARPROFEN;kanujofen;carprofeno;rac Carprofen;Carprofen CRS
- CBNumber:
- CB0663159
- Molecular Formula:
- C15H12ClNO2
- Molecular Weight:
- 273.71
- MDL Number:
- MFCD00079028
- MOL File:
- 53716-49-7.mol
| Melting point | 186-1880C |
|---|---|
| Boiling point | 509.1±35.0 °C(Predicted) |
| Density | 1.2011 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| form | powder |
| pka | 4.84±0.30(Predicted) |
| color | white to beige |
| Merck | 14,1862 |
| InChI | InChI=1S/C15H12ClNO2/c1-8(15(18)19)9-2-4-11-12-7-10(16)3-5-13(12)17-14(11)6-9/h2-8,17H,1H3,(H,18,19) |
| InChIKey | PUXBGTOOZJQSKH-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=C(Cl)C=C2)C2=C1C=C(C(C)C(O)=O)C=C2 |
| CAS DataBase Reference | 53716-49-7(CAS DataBase Reference) |
| FDA UNII | FFL0D546HO |
| EPA Substance Registry System | 9H-Carbazole-2-acetic acid, 6-chloro-.alpha.-methyl- (53716-49-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301 | |||||||||
| Precautionary statements | P301+P330+P331+P310 | |||||||||
| Hazard Codes | T,Xn | |||||||||
| Risk Statements | 25-36/37/38-20/21/22 | |||||||||
| Safety Statements | 45-36-26 | |||||||||
| RIDADR | UN 2811 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | FE3180000 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29339900 | |||||||||
| Toxicity | LD50 orally in mice: 400 mg/kg (Berger, Corraz) | |||||||||
| NFPA 704 |
|
Carprofen price More Price(35)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 33975 | Carprofen VETRANAL?, analytical standard | 53716-49-7 | 100mg | $119 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1096699 | Carprofen United States Pharmacopeia (USP) Reference Standard | 53716-49-7 | 200mg | $436 | 2024-03-01 | Buy |
| TCI Chemical | C2701 | Carprofen >98.0%(HPLC)(T) | 53716-49-7 | 1g | $82 | 2024-03-01 | Buy |
| TCI Chemical | C2701 | Carprofen >98.0%(HPLC)(T) | 53716-49-7 | 5g | $246 | 2024-03-01 | Buy |
| Cayman Chemical | 16409 | Carprofen ≥98% | 53716-49-7 | 1g | $70 | 2024-03-01 | Buy |
Carprofen Chemical Properties,Uses,Production
Description
Carprofen is a non-steroidal anti-inflammatory drug (NSAID) commonly used in animals to combat pain and inflammation, particularly as associated with osteoarthritis. Like many NSAIDs, carprofen inhibits both cyclooxygenases COX-1 and COX-2 (IC50s = 22.3 and 3.9 μM, respectively). It also inhibits fatty acid amide hydrolase (IC50 = 74 μM), blocking the metabolism of the cannabinoid receptor ligand, arachidonoyl ethanolamide .
Chemical Properties
Off-White Crystalline Solid
Originator
Imadyl,Roche,Switz.,1981
History
Carprofen has strong anti-inflammatory and analgesic activity. It has been used in human medicine for more than 10 years at doses of 150 to 600 mg per day. In human clinical trials, Carprofen was generally well tolerated. Most adverse reactions were transient and mild, such as gastrointestinal discomfort or pain and nausea. The incidence of side effects in humans is similar to that of aspirin and other NSAIDs. However, for commercial reasons, carprofen has been withdrawn from the market and is no longer available for human use.
Uses
Carprofen is a non steroidal anti-inflammatory that is used by veterinarians for the relief of arthritic systems in dogs. It can be used for joint pain or post operative inflammation. Carprofen, is al so used for the relief from pain and inflammation associated with osteoarthritis, hip dysplasia and other joint issues.
Uses
antiinflammatory, analgesic
Uses
Carprofen is a non-steroidal anti-inflammatory drug (NSAID) commonly used in animals to combat pain and inflammation, particularly as associated with osteoarthritis. Like many NSAIDs, carprofen inhibits both cyclooxygenases COX-1 and COX-2 (IC50s = 22.3 and 3.9 μM, respectively). It also inhibits fatty acid amide hydrolase (IC50 = 74 μM), blocking the metabolism of the cannabinoid receptor ligand, arachidonoyl ethanolamide .
Definition
ChEBI: Propanoic acid in which one of the methylene hydrogens is substituted by a 6-chloro-9H-carbazol-2-yl group. A non-steroidal anti-inflammatory drug, it is no longer used in human medicine but is still used for treatment of arthritis in elderly dogs.
Manufacturing Process
A mixture of 34.9 g of 6-chloro-α-methyl-1,2,3,4-tetrahydrocarbazole-2-acetic
acid ethyl ester (mixture of diastereomers), 350 ml CP xylene and 56.0 g of
p-chloranil was stirred and heated under an atmosphere of dry nitrogen. The
reaction flask was wrapped in aluminum foil in order to keep out any
extraneous light. After the reaction mixture had stirred at reflux temperature
for 6 hours, heating and stirring were stopped and the reaction mixture was
left overnight at room temperature. The supernatant liquid was decanted through a filter. The residue was triturated with 100 ml of warm benzene and
the supernatant liquid was decanted through a filter. This process was
repeated three more times. Ether (300 ml) was added to the combined
filtrates. The solution was extracted with cold 2 N sodium hydroxide (3 x 100
ml), washed by extraction with water until neutral and dried over anhydrous
magnesium sulfate. Following filtration of the desiccant and evaporation of the
solvent, a residue of 35.5 g remained. Crystallization from 50 ml of methanol
gave 14.8 g of 6-chloro-α-methylcarbazole-2-acetic acid ethyl ester, MP 106°-
107.5°C (43.2%).
A stirred mixture of 11 g of 6-chloro-α-methylcarbazole-2-acetic acid ethyl
ester, 100 ml ethanol and 100 ml of 3 N sodium hydroxide was heated (N2
atmosphere). After 2 hours at reflux, the reaction mixture was concentrated
to dryness under reduced pressure. Water (300 ml) and ice (200 g) were
added to the residue and concentrated hydrochloric acid was added until the
mixture was strongly acid. The acidic mixture was extracted with ether (3 x
200 ml). The ether extracts were combined, washed by extraction with water
(3 x 100 ml) and dried over anhydrous magnesium sulfate. Following filtration
of the desiccant and evaporation of the solvent, a yield of 9.89 (98.2%) was
obtained. Crystallization from CHCl3 yielded 6.2 g (62.0%) of 6-chloro-α-
methylcarbazole-2-acetic acid, MP 197°-198°C. A second crop of 1.6 g, MP
195°-199°C was obtained from the mother liquors.
Therapeutic Function
Antiinflammatory
Mechanism of action
Carprofeng is a non-narcotic NSAID with analgesic and antipyretic properties. It acts in the same way as most NSAIDs in that Carprofeng acts by inhibiting cyclooxygenase (COX), which selectively inhibits COX-2, thereby inhibiting the release of several prostaglandins involved in the chronic inflammatory response that is thought to be present in canine OA.
Side effects
Common side effects of Carprofeng in dogs include vomiting, diarrhoea, loss of appetite, constipation, behavioural changes; and in severe cases, black stools, gastrointestinal ulcers and gastrointestinal haemorrhage, jaundice, skin changes, involuntary muscle movements, and kidney damage (increased thirst, increased urination, and refusal to eat).
Veterinary Drugs and Treatments
Carprofen is labeled (in the USA) for the relief of pain and inflammation
in dogs. It may also prove to be of benefit in other species as
well, but data is scant to support its safety beyond very short-term
use at this time. In Europe, carprofen is reportedly registered for
single dose use in cats, but there have been reported problems (e.g.,
vomiting) with cats receiving more than a single dose.
Carprofen is being investigated for antineoplastic effects in dogs
and may be a useful adjunctive treatment for some types of tumors
with COX-2 overexpression.
Carprofen Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Xi’an Sengmei Biotech Co.,Ltd. | +8615398038360 | lionel@accenturebio.com | China | 288 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Zibo Shiji Zhonglian Medical Technology Co., Ltd. | 15069388126 15069388126 | sales@sjzlpharm.com | China | 61 | 58 |
| Xiamen Wonderful Bio Technology Co., Ltd. | +8613043004613 | Sara@xmwonderfulbio.com | China | 305 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 616 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 | 2691956269@qq.com | China | 359 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 | sarah@tnjone.com | China | 874 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
Related articles
- Non-steroidal anti-inflammatory drug Carprofen: Uses and Adverse reactions
- Carprofen is a non-steroidal anti-inflammatory drug (NSAID), this class of drugs also includes Ibuprofen, Naproxen and Ketopro....
- Mar 8,2024
View Lastest Price from Carprofen manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-05 | Carprofen
53716-49-7
|
US $0.00 / kg | 1kg | 99% | 1000kg | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2024-03-16 | Carprofen
53716-49-7
|
US $0.00-0.00 / KG | 1KG | 99 | 1000tons | Shanghai Affida new material science and technology center | |
 |
2024-03-08 | Carprofen
53716-49-7
|
US $10.00 / kg | 1kg | 99% | 1000kg | Nantong Guangyuan Chemicl Co,Ltd |
53716-49-7(Carprofen)Related Search:
1of4