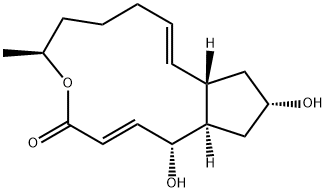
Brefeldin A
- CAS No.
- 20350-15-6
- Chemical Name:
- Brefeldin A
- Synonyms
- BFA;CS-766;CYANEIN;DECUMBIN;cyanaein;ASCOTOXIN;Brefeldin;NSC 89671;NECTROLIDE;NSC 107456
- CBNumber:
- CB0689753
- Molecular Formula:
- C16H24O4
- Molecular Weight:
- 280.36
- MDL Number:
- MFCD12913297
- MOL File:
- 20350-15-6.mol
- MSDS File:
- SDS
| Melting point | 200-205 °C |
|---|---|
| Boiling point | 492.7±45.0 °C(Predicted) |
| alpha | 93 º (C=2 IN MEOH) |
| Density | 1.108±0.06 g/cm3(Predicted) |
| vapor pressure | 0.56 hPa ( 20 °C) |
| Flash point | 87 °C |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | methanol: 10 mg/mL, clear, colorless to faintly yellow |
| pka | 12.92±0.60(Predicted) |
| form | Crystalline Powder |
| color | White to almost white |
| Water Solubility | Soluble in dimethylsulfoxide, dichloromethane and ethanol. Slightly soluble in water. |
| Merck | 13,1355 |
| BRN | 25191 |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| InChIKey | QSECOIOBNZLGOD-OBCVZTHZSA-N |
| CAS DataBase Reference | 20350-15-6 |
| FDA UNII | XG0D35F9K6 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301 | |||||||||
| Precautionary statements | P301+P310 | |||||||||
| Hazard Codes | Xn,T | |||||||||
| Risk Statements | 22-25-36/37/38-20/21/22 | |||||||||
| Safety Statements | 24/25-45-36-26 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GY8410000 | |||||||||
| Autoignition Temperature | 301 °C | |||||||||
| HS Code | 29411090 | |||||||||
| Toxicity | LD50 i.p. in mice: >200 mg/kg (Haerri) | |||||||||
| NFPA 704 |
|
Brefeldin A price More Price(74)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | B6542 | Brefeldin A ≥99% (HPLC and TLC), BioXtra, for molecular biology | 20350-15-6 | 5mg | $210 | 2024-03-01 | Buy |
| Sigma-Aldrich | B5936 | Brefeldin A from Penicillium brefeldianum, Ready Made Solution, 10?mg/mL in DMSO, 0.2 μm filtered | 20350-15-6 | 200μL | $161 | 2024-03-01 | Buy |
| Sigma-Aldrich | 5.00583 | InSolution (+)-Brefeldin A, Eupenicillium brefeldianum - CAS 20350-15-6 - Calbiochem | 20350-15-6 | 2mg | $142 | 2024-03-01 | Buy |
| TCI Chemical | B3820 | Brefeldin A | 20350-15-6 | 10MG | $127 | 2024-03-01 | Buy |
| TCI Chemical | B3820 | Brefeldin A | 20350-15-6 | 50MG | $436 | 2024-03-01 | Buy |
Brefeldin A Chemical Properties,Uses,Production
Description
Brefeldin A (20350-15-6) is a specific inhibitor of protein translocation from endoplasmic reticulum to Golgi. Induces apoptosis. Brefeldin A activates sphingomyelin metabolism. Cell permeable.
Chemical Properties
White Powder
Uses
Brefeldin A (BFA) is a natural fungal metabolite which has been used extensively to study intracellular transport by vesicles or endosomes. Early studies demonstrated that BFA reversibly interferes with protein trafficking and secretion mediated by the Golgi apparatus and endoplasmic reticulum. BFA directly and reversibly inhibits Sec7 domain-containing guanine-exchange factors which are necessary for ADP-ribosylation factor activation associated with vesicular transport (IC50 = ~10 μM). BFA is used to study endosomal trafficking and function in cells of plants as well as those of fungi, invertebrates, and vertebrates.
Uses
A macrolide isolated from Penicillium brefeldianum. It affects the vesticular transport of the Golgi apparatus and induces DNA fragmentation which leads to apoptosis
Uses
Brefeldin A is a potent inhibitor of cell growth first described in 1958, then independently rediscovered by several groups as a potent active in a broad range of bioassays. Brefeldin has antiviral, antibiotic, antifungal, antitumour and herbicidal activity. Early studies on the mode of action of brefeldin identified inhibition of protein and nucleic acid synthesis by disruption of the Golgi apparatus. The precise molecular target is a subset of Sec7-type GTP exchange factors (GEFs) that activate a small GTPase, Arf1p, an integral component of protein trafficking and signalling.
Definition
ChEBI: A metabolite from Penicillium brefeldianum that exhibits a wide range of antibiotic activity.
General Description
Specifically and reversibly blocks translocation of proteins from the endoplasmic reticulum (ER) to the Golgi apparatus without affecting endocytosis or lysosome function. Inhibitor of HDL-mediated cholesterol efflux. Causes disassembly of the Golgi complex and ER swelling in a variety of mammalian cell lines at <40 ng/ml. Blocks binding of ADP-ribosylation factor to the Golgi and inhibits the GDP-GTP exchange. Inhibits the activity of BIG1 and BIG2, the guanine-nucleotide exchange proteins for ADP-ribosylation factors. Also available as a 25 mM solution in DMSO (Cat. No. 500583).
Biological Activity
Reversible inhibitor of protein translocation from the endoplasmic reticulum (ER) to the Golgi apparatus. Blocks binding of ADP-ribosylation factor to the Golgi apparatus and inhibits GDP-GTP exchange.
Biochem/physiol Actions
Primary TargetBlocks translocation of proteins from the endoplasmic reticulum (ER) to the Golgi apparatus
storage
-20°C (desiccate)
Purification Methods
Brefeldin A was isolated from Penicillium brefeldianum and recrystallised from aqueous MeOH/EtOAc or MeOH. Its solubility in H2O is 0.6mg/mL, 10mg/mL in MeOH and 24.9mg/mL in EtOH. The O-acetate recrystallises from Et2O/pentane and has m 130-131o, [] D +17o (c 0.95, MeOH). [Sigg Helv Chim Acta 47 1401 1964, UV and IR: H.rri et al. Helv Chim Acta 46 1235 1963, total synthesis: Kitahara et al. Tetrahedron 3021 1979, X-ray : Weber et al. Helv Chim Acta 54 2763 1971, Beilstein 18 III/IV 1220.]
References
1) Fujiwara et al. (1988) Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum; J. Biol. Chem., 263 18545 2) Shao et al. (1996), Brefeldin A is a potent inducer of apoptosis in human cancer cells independently of p53; Exp. Cell Res., 227 190 3) Linardic et al. (1996), Brefeldin A promotes hydrolysis of sphingomyelin; Cell Growth Differ., 7 765
Brefeldin A Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Fuxin Pharmaceutical | +86-021-021-50872116 +8613122107989 | contact@fuxinpharm.com | China | 10297 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| HubeiwidelychemicaltechnologyCo.,Ltd | 18627774460 | faith@widelychemical.com | CHINA | 742 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shenzhen Excellent Biotech Co., Ltd. | 13480692018 | ramyan@ex-biotech.com | CHINA | 954 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
View Lastest Price from Brefeldin A manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-09 | brefeldin
20350-15-6
|
US $0.00-0.00 / Kg | 1Kg | 99.9% | 200tons | airuikechemical co., ltd. | |
 |
2023-09-20 | Brefeldin A
20350-15-6
|
US $10.00-1.00 / kg | 1kg | 0.99 | 20 tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-09-06 | Brefeldin A
20350-15-6
|
US $0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. |
-

- brefeldin
20350-15-6
- US $0.00-0.00 / Kg
- 99.9%
- airuikechemical co., ltd.
-

- Brefeldin A
20350-15-6
- US $10.00-1.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- Brefeldin A
20350-15-6
- US $0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
20350-15-6(Brefeldin A)Related Search:
1of4