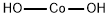Cobalt(II) hydroxide
- CAS No.
- 21041-93-0
- Chemical Name:
- Cobalt(II) hydroxide
- Synonyms
- COBALT HYDROXID;t(II) hydroxide;Cobalt hydroxide;cobaltdihydroxide;Cobalt(II)hydroxi;cobaltoushydroxide;cobalt(2+)hydroxide;cobalt (Ⅱ) hydroxide;COBALT(II) HYDROXIDE;Cobalt(II)dihydoxide
- CBNumber:
- CB0716301
- Molecular Formula:
- CoH2O2
- Molecular Weight:
- 92.95
- MDL Number:
- MFCD00016015
- MOL File:
- 21041-93-0.mol
- MSDS File:
- SDS
| Melting point | °Cd ec.) |
|---|---|
| Density | 3.597 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | slightly soluble in H2O; soluble in acid solutions |
| form | Powder |
| Specific Gravity | 3.597 |
| color | Pink to purple |
| PH | 9.15(1 mM solution);9.15(10 mM solution);9.15(100 mM solution) |
| Water Solubility | Soluble in acids and ammonia. Very slightly soluble in water. Insoluble in dilute alkalis. |
| Sensitive | Air Sensitive |
| Merck | 14,2442 |
| Solubility Product Constant (Ksp) | pKsp: 14.23 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3 |
| Stability | Stability |
| CAS DataBase Reference | 21041-93-0(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 51B9AYG60K |
| EPA Substance Registry System | Cobalt hydroxide (Co(OH)2) (21041-93-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H317-H319-H330-H334-H350-H360-H410 | |||||||||
| Precautionary statements | P273-P280-P301+P312-P302+P352-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,N | |||||||||
| Risk Statements | 20/21/22-36/37/38-50/53-42/43-40-20/22 | |||||||||
| Safety Statements | 26-37/39-36 | |||||||||
| RIDADR | UN 3077 9 / PGIII | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GG0904500 | |||||||||
| TSCA | Yes | |||||||||
| NFPA 704 |
|
Cobalt(II) hydroxide price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 342440 | Cobalt(II) hydroxide technical grade, 95% | 21041-93-0 | 250g | $108 | 2024-03-01 | Buy |
| Alfa Aesar | 012524 | Cobalt(II) hydroxide, 99.9% (metals basis) | 21041-93-0 | 100g | $54.3 | 2023-06-20 | Buy |
| Alfa Aesar | 012524 | Cobalt(II) hydroxide, 99.9% (metals basis) | 21041-93-0 | 2kg | $821 | 2023-06-20 | Buy |
| Strem Chemicals | 93-2741 | Cobalt(II) hydroxide (97%-Co) | 21041-93-0 | 50g | $46 | 2024-03-01 | Buy |
| Strem Chemicals | 93-2741 | Cobalt(II) hydroxide (97%-Co) | 21041-93-0 | 250g | $184 | 2024-03-01 | Buy |
Cobalt(II) hydroxide Chemical Properties,Uses,Production
Physical Properties
Two forms occur, a rose-red powder (more stable) and a bluish-green powder less stable than the red form; rhombohedral crystals; density 3.597 g/cm3; decomposes on heating; practically insoluble in water 3.2 mg/L; Ksp 1.0x10–15; soluble in acids and ammonia; insoluble in dilute alkalis.

The addition of alkali metal hydroxides to solutions of cobalt(II) salts results in the precipitation of cobalt(II) hydroxide in either a blue or pink form depending upon the conditions. The pink form is the more stable of the two and is obtained when a suspension of the blue form is allowed to stand or is warmed. Cobalt(II) hydroxide is amphoteric, dissolving in alkalis to form blue solutions of the [Co(OH)4]2~ ions. In the presence of alkali, suspensions of Co(OH)8 are oxidized by air to the brown CoO(OH), this oxidation being brought about rapidly by oxidants such as hypochlorite, bromine water or hydrogen peroxide. The pink Co(OH)2 (density 3.597) has the brucite (Mg(OH)2) structure in which the cobalt atoms are surrounded by six hydroxides. The blue form is more disordered, but its structure is not known for certain.
Uses
Cobalt(II) hydroxide is used as a drier for paints and varnishes and is added to lithographic printing inks to enhance their drying properties. Other applications are in the preparation of cobalt salts; as a catalyst; and in storage battery electrodes.
Preparation
Cobalt(II) hydroxide is obtained as a precipitate when an alkaline hydroxide is added to an aqueous solution of cobalt(II) salt:
CoCl2 + 2NaOH → Co(OH)2 + 2NaCl
Co(NO3)2 + 2NaOH → Co(OH)2 + 2NaNO3
Reactions
Thermal decomposition to cobaltous oxide, CoO, occurs at 168°C in a vacuum.
Cobalt(II) hydroxide is oxidized by air and other oxidizing agents, forming cobalt(III) hydroxide, Co(OH)3. Reactions with mineral acids produce corresponding Co2+ salts.
Description
The addition of alkali metal hydroxides to solutions of cobalt(II) salts results in the precipitation of cobalt(II) hydroxide in either a blue or pink form depending upon the conditions. The pink form is the more stable of the two and is obtained when a suspension of the blue form is allowed to stand or is warmed. Cobalt(II) hydroxide is amphoteric, dissolving in alkalis to form blue solutions of the [Co(OH)4]2- ions. In the presence of alkali, suspensions of Co(OH) are oxidized by air to the brown CoO(OH), this oxidation being brought about rapidly by oxidants such as hypochlorite, bromine water or hydrogen peroxide. The pink Co(OH)2 (density 3.597) has the brucite (Mg(OH)2) structure in which the cobalt atoms are surrounded by six hydroxides. The blue form is more disordered, but its structure is not known for certain.
Chemical Properties
Rose-red powder.Soluble in acids and ammonium salt solutions; insoluble in water and alkalies.
Uses
Cobalt salts, paint and varnish driers, catalyst, storage-battery electrodes.
Uses
Cobalt(II) hydroxide is used as a drying agent for paints and varnishes. It acts as an aid to dry the lithographic inks and catalyst in the preparation of battery electrode impregnating solutions. It is also involved in the synthesis of other cobalt compounds.
Flammability and Explosibility
Not classified
Cobalt(II) hydroxide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 | dominicguo@gk-bio.com | CHINA | 9427 | 58 |
View Lastest Price from Cobalt(II) hydroxide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-06 | Cobalt(II) hydroxide
21041-93-0
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2023-05-19 | Cobalt(II) hydroxide
21041-93-0
|
US $5.00-2.00 / KG | 1KG | 99% | 10000kg | Hebei Guanlang Biotechnology Co,.LTD | |
 |
2021-08-12 | Cobalt hydroxide
21041-93-0
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Cobalt(II) hydroxide
21041-93-0
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- Cobalt(II) hydroxide
21041-93-0
- US $5.00-2.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co,.LTD
-

- Cobalt hydroxide
21041-93-0
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
21041-93-0(Cobalt(II) hydroxide)Related Search:
1of4