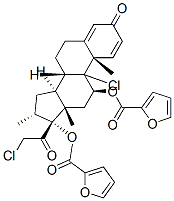
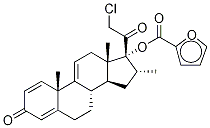
Mometasone furoate
- CAS No.
- 83919-23-7
- Chemical Name:
- Mometasone furoate
- Synonyms
- NASONEX;Mometason Furoate;MOMETASONE 17-FUROATE;(11β,16α)-9,21-Dichloro-17-[(2-furanylcarbonyl)oxy]-11-hydroxy-16-Methyl-pregna-1,4-diene-3,20-dione;Ecural;ElocoM;EloMet;Monovo;AsManex;SCH-32088
- CBNumber:
- CB0725569
- Molecular Formula:
- C27H30Cl2O6
- Molecular Weight:
- 521.43
- MDL Number:
- MFCD00866003
- MOL File:
- 83919-23-7.mol
- MSDS File:
- SDS
| Melting point | 218-220°C |
|---|---|
| alpha | D26 +58.3° (dioxane) |
| Boiling point | 655.5±55.0 °C(Predicted) |
| Density | 1.37±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥20mg/mL |
| pka | 13.02±0.70(Predicted) |
| form | powder |
| color | white to off-white |
| optical activity | [α]/D +50 to +60°, c = 0.5 in methanol |
| InChIKey | WOFMFGQZHJDGCX-ZULDAHANSA-N |
| SMILES | C[C@]12C[C@H](OC(=O)C3=CC=CO3)C3([C@](CCC4[C@]3(C)C=CC(=O)C=4)([H])[C@]1([H])C[C@@H](C)[C@]2(OC(=O)C1OC=CC=1)C(=O)CCl)Cl |&1:1,3,13,17,25,28,30,r| |
| CAS DataBase Reference | 83919-23-7(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | mometasone furoate; Nasonex |
| FDA UNII | 04201GDN4R |
| NCI Drug Dictionary | mometasone furoate |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P337+P313-P362-P403+P233-P405-P501 | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 29372290 | |||||||||
| NFPA 704 |
|
Mometasone furoate price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | M4074 | Mometasone furoate ≥98% (HPLC) | 83919-23-7 | 5mg | $82.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1445470 | Mometasone furoate United States Pharmacopeia (USP) Reference Standard | 83919-23-7 | 200mg | $436 | 2024-03-01 | Buy |
| Cayman Chemical | 21365 | Mometasone Furoate ≥98% | 83919-23-7 | 25mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 21365 | Mometasone Furoate ≥98% | 83919-23-7 | 50mg | $61 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR1400 | Mometasone Furoate Pharmaceutical Secondary Standard; Certified Reference Material | 83919-23-7 | 500mg | $116 | 2024-03-01 | Buy |
Mometasone furoate Chemical Properties,Uses,Production
Description
Mometasone furoate is a topical steroidal antiinflammatory useful in the treatment of corticosteroid-responsive dermatoses. As a steroidal antiinflammatory of grade II potency, mometasone furoate is characterized by a once-daily dose regimen and a relatively wide safety margin.
Chemical Properties
White-to-Off-White Solid
Originator
Schering-Plough (USA)
Uses
Mometasone furoate is a deuterated topical corticosteroid used as an anti-inflammatory. Mometasone furoate has been used to study its effect on Leishmania major amastigotes. It could as labeled Mometasone, intended for use as an internal standard for the quantification of Mometasone by GC- or LC-mass spectrometry. Formulations containing mometasone furoate have been used in the treatment of allergic rhinitis, atopic dermatitis, and asthma. Intranasal administration of mometasone furoate (1 μg/nostril) reduces the frequency of sneezing and nasal rubbing in a rat model of histamine-induced allergic rhinitis. Topical application of mometasone furoate reduces croton oil-induced ear edema in mice (ED50 = 0.31 μg/ml).
Definition
ChEBI: Mometasone furoate is a 2-furoate ester, a steroid ester, an 11beta-hydroxy steroid, a 20-oxo steroid, an organochlorine compound and a 3-oxo-Delta(1),Delta(4)-steroid. It has a role as an anti-inflammatory drug and an anti-allergic agent. It is functionally related to a mometasone.
Manufacturing Process
METHOD I (Patent U.S. 4,472,393)
A. 21-Chloro-9β,11β-epoxy-17α-hydroxy-16α-methyl-1,4-pregnadiene-3,20-
dione
Prepare a solution of 5.0 g. of 9β,11β-epoxy-17α,21-dihydroxy-16α-methyl-
1,4-pregnadiene-3,20-dione in 20 ml of dry pyridine. Cool on an ice bath; to
the stirred solution under nitrogen, add dropwise 1.1 ml of mesyl chloride.
Remove the ice bath and continue stirring at room temperature for 30 min.
Add 2.0 g of lithium chloride and continue stirring for a further 150 min. Add
to a mixture of 150 ml ethyl acetate and 100 ml distilled water. Wash the
organic phase with dilute 3% aqueous hydrochloric acid, then saturated
aqueous sodium chloride solution and finally saturated sodium bicarbonate
solution. Dry the organic phase over magnesium sulfate, filter and remove the
solvent to give 4.62 g of 21-chloro-9β,11β-epoxy-17α-hydroxy-16α-methyl-
1,4-pregnadiene-3,20-dione.
B. 21-Chloro-9β,11β-epoxy-17α-hydroxy-16α-methyl-1,4-pregnadiene-3,20-
dione 17-(2'-furoate)
Prepare under argon a solution of 8 g of 4-dimethylaminopyridine in 250 ml of
dry methylene chloride. Cool on an ice bath and add to the stirred solution 6.0
ml of 2-furoyl chloride. Remove from the ice bath, allow the temperature to
rise to room temperature and then add 11.5 g of the 21-chloro-9β,11β-epoxy-
17α-hydroxy-16α-methyl-1,4-pregnadiene-3,20-dione. After 24 hours add 500
ml of ethyl acetate saturated with water. Filter off the precipitate and then
evaporate off the solvent to give the crude 21-chloro-9β,11β-epoxy-17α-
hydroxy-16α-methyl-1,4-pregnadiene-3,20-dione 17-(2'-furoate).
C. 9α,21-Dichloro-11β,17α-hydroxy-16α-methyl-1,4-pregnadiene-3,20-dione
17-(2'-furoate)
To the crude 21-chloro-9β,11β-epoxy-17α-hydroxy-16α-methyl-1,4-
pregnadiene-3,20-dione 17-(2'-furoate) add 50 ml of glacial acetic acid, then add a solution of 3.5 g of anhydrous hydrogen chloride in 125 ml of glacial
acetic acid. Stir for 15 minutes and then quench with 500 ml of distilled water.
Filter off the solids, recrystallise from methanol:water, dry for 24 hours under
vacuum to give 12.6 g 9α,21-dichloro-11β,17α-hydroxy-16α-methyl-1,4-
pregnadiene-3,20-dione 17-(2'-furoate) (yield 83% of theory).
Prepare under nitrogen a solution of 1.80 g of 21-chloro-17α-hydroxy-16α-
methyl-1,4,9(11)-pregnatriene-3,20-dione 17-(2'-furoate). Add, with stirring,
a solution of 1.15 ml of 70% perchloric acid in 2.53 ml of distilled water, and
immediately thereafter 604 mg of 1,3-dichloro-5,5-dimethylhydantoin. Stir the
reaction mixture for twenty minutes and then raise the temperature to
ambient temperature. Monitor the consumption of starting material by thin
layer chromatography of aliquots using chloroform:1,3-dichloro-5,5-
dimethylhydantoinyl acetate (9:1) and hexane:ethyl acetate (1:1). When the
starting material is consumed, pour the reaction mixture into 500 ml of
distilled water containing the 1,3-dichloro-5,5-dimethylhydantoin and 7 g of
sodium bisulphite. Add sodium chloride until the solution is saturated. Filter
the precipitated solid, wash and dry at 50°C under vacuum. Purify the
resulting crude product by preparative chromatography on 1000 micron silica
gel plates using chloroform: ethyl acetate (19:1). Elute the desired band with
ethyl acetate, filter the eluate and evaporate at room temperature to give
crude product (1.3 g). Recrystallize the product by dissolving in refluxing
methylene chloride, filtering and then replacing the methylene chloride at
reflux with methanol and then the methanol with distilled water. Cool the
suspension to room temperature, filter and dry under vacuum at 50°C to give
the pure pregna-1,4-diene-3,20-dione, 9,21-dichloro-17-((2-
furanylcarbonyl)oxy)-11-hydroxy-16-methyl-, (11β-,16α)-.
METHOD II
The present invention (Patent U.S. 6,177,560) refers to a new process for the
preparation of mometasone furoate carried out by esterifiication of the 17
hydroxy group of mometasone without prior protection of the 11 hydroxy
group. Mometasone (30 g) was suspended in methylene chloride (300 ml) and
the resulting suspension was cooled to 0-5°C. At this temperature
triethylamine (57 ml) was added. 2-Furoyl chloride (24 ml) was then added
slowly. The mixture was then stirred at 8-12°C until the level of mometasone
present was lower than 0.2% by HPLC. The reaction solution was then cooled
to between -5-5°C and water (120 ml) was added with stirring. After stirring
for 1 hour at 10-15°C the mixture was cooled to between 0-5°C and
concentrated hydrochloric acid was added to adjust the pH of the aqueous
layer between 1 and 2.
The phases were separated and the aqueous layer was extracted with
methylene chloride (60 ml). To the combined organic layers concentrated
hydrochloric acid (90 ml) and acetic acid (30 ml) was added at a temperature
15-25°C. Then the two phase reaction mixture was stirred until less than
0.1% of the side products remained as monitored by HPLC. The reaction
mixture was cooled to 0-5°C and water (120 ml) was added. The lower
organic layer was separated, water (120 ml) and 8 N aqueous sodium
hydroxide solution (about 30 ml) were added to adjust the pH to between 5
and 6. After stirring for 2 hours the organic layer was separated and washed
with water (120 ml). The organic solution [containing the mometasone 17-(2-
furoate)] was concentrated by distillation to a volume of 120 ml. Further methanol (120 ml) was added and the mixture was concentrated to 120 ml.
This procedure was repeated twice more. The reaction mixture was slowly
cooled to 0-5°C and stirred for 2 hours. The crude mometasone 17-(2-
furoate) was then filtered off and washed with cold methanol.
Purification of mometasone 17-(2-furoate)
The wet cake was dissolved in acetone (395 ml) and charcoal (3 g) was
added. After 24 hours, the charcoal was filtered off and washed with acetone
(90 ml). Charcoal (3 g) was added to the solution and the solution stirred for
at least 24 hours at between 15-25°C. The charcoal was then filtered off and
washed with acetone (75 ml). The solution was concentrated by distillation to
a volume of 120 ml. During this concentration the mometasone 17-(2-furoate)
started to crystallise. Methanol (120 ml) was added and the solution was
again concentrated to 120 ml. This procedure was repeated twice. The
suspension was cooled slowly to 0-5°C and stirred for about 2 hours at this
temperature. The pure mometasone 17-(2-furoate) was then filtered off and
washed with cold methanol. The product was dried at 60-70°C. A yield of
29.92 g was obtained.
brand name
Asmanex (Schering);Elocon (Schering).
Therapeutic Function
Glucocorticoid
Biological Functions
Mometasone furoate has strong local anti-inflammatory activity equivalent to that of fluticasone propionate. It has a quick onset of action relative to the other inhaled/intranasal steroids with the least systemic availability and, consequently, the fewest systemic side effects.
General Description
Mometasone furoate (Asmanex,Nasonex) undergoes extensive metabolism to multiplemetabolites. No major metabolites are detectable in humanplasma after oral administration, but the 6β-hydroxy metaboliteis detectable by use of human liver microsomes. Thismetabolite is formed via the CYP3A4 pathway.
General Description
Mometasone furoate,9,21-dichloro-17α-[(2-furanylcarbonyl)oxy]-11β-hydroxy-16α-methylpregna-1,4-diene-3,20-dione (Elocon), is a highpotencyGC available in cream, lotion, or ointment formulationsfor topical use. In addition, mometasone furoatemonohydrate is formulated for treating allergic rhinitis andasthma.
Biochem/physiol Actions
Mometasone furoate is a 17-heterocyclic intranasal corticosteroid. It is effectively used as a first-line daily intranasal therapeutic for allergic rhinitis and nasal polyposis. Mometasone furoate is also used as an adjunct to anti-bacterials for treating acute rhinosinusitis. In addition, it relieves the symptoms of asthma in both adults and adolescents by exhibiting a broad spectrum of anti-inflammatory properties.
Mechanism of action
Mometasone furoate is the most recent glucocorticoid to be developed and commercialized. It has a number of unique functional groups that confer enhanced glucocorticoid activity as well as pharmacokinetic advantages. The combination of the C-21 chloro and the furoic acid ester at C-17 results in the highest glucocorticoid receptor affinity of any topical corticosteroid. The 16α- methyl decreases the mineralocorticoid effects, and the 9α-chloro group increases both glucocorticoid and mineralocorticoid activities. Inhaled mometasone furoate acts locally as an anti-inflammatory treatment for asthma and has the least systemic bioavailability of all the inhaled glucocorticoids (<1%).
Clinical Use
Mometasone furoate was originally marketed as a topically applied corticosteroid, but because of its low systemic bioavailability, it was found to be more useful in the treatment of allergic disorders and lung diseases (107).
Metabolism
It is extensively metabolized, with 6β-hydroxymometasone and 21-hydroxymometasone being found among the metabolites. Inhaled mometasone furoate in mainly excreted in the feces (~74%) as metabolites and only minimally excreted in the urine (~8%). It has a relatively long half-life in the lung and is administered once daily, usually in the evening.
Mometasone furoate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Jewim Pharmaceutical (Shandong) Co., Ltd. | +86-15552509998 +86-15621883869 | liutf@jewim.com.cn | China | 251 | 58 |
| Nanjing Shizhou Biotechnology Co., Ltd | +86-15850508050 +86-15850508050 | sean.lv@synzest.com | China | 323 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Zibo Shiji Zhonglian Medical Technology Co., Ltd. | 15069388126 15069388126 | sales@sjzlpharm.com | China | 61 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| Rixing Chemical CO.,LTD | +86-13237129059 -13237129059 | sales@rixingchemi.com | CHINA | 229 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
Related articles
- Mometasone furoate for the treatment of asthma
- Mometasone furoate is a potent glucocorticoid that reduces inflammation and has high efficacy in treating asthma, especially w....
- Jan 30,2024
- A widely used anti-inflammatory agent-Mometasone furoate
- Mometasone furoate is an anti-inflammatory drug that can be used in the treatment of a variety of diseases.
- Sep 19,2023
View Lastest Price from Mometasone furoate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-16 | Mometasone furoate
83919-23-7
|
US $0.00 / KG | 100g | 98%+ | 100kg | WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD | |
 |
2024-01-02 | Mometasone furoate
83919-23-7
|
US $90.00-900.00 / kg | 10kg | 0.99 | 20tons | Zibo Hangyu Biotechnology Development Co., Ltd | |
 |
2023-11-30 | Mometasone furoate
83919-23-7
|
US $1.00 / KG | 5KG | 99.5% | 100 | Zibo Shiji Zhonglian Medical Technology Co., Ltd. |
-

- Mometasone furoate
83919-23-7
- US $0.00 / KG
- 98%+
- WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD
-

- Mometasone furoate
83919-23-7
- US $90.00-900.00 / kg
- 0.99
- Zibo Hangyu Biotechnology Development Co., Ltd
-

- Mometasone furoate
83919-23-7
- US $1.00 / KG
- 99.5%
- Zibo Shiji Zhonglian Medical Technology Co., Ltd.
83919-23-7(Mometasone furoate)Related Search:
1of4