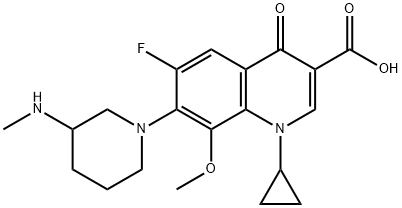
Balofloxacin
- CAS No.
- 127294-70-6
- Chemical Name:
- Balofloxacin
- Synonyms
- q35;BALOFLOXACIN;Barlow of sand;BALOFLOXACIN 98.5%;Balofloxacin425.46;Balofloxacin, >=99%;Balofloxacin hydrate;Balofloxacin USP/EP/BP;Cypermethrin Impurity 4;BALOFLOXACIN 98.5% EXTRA PURE
- CBNumber:
- CB0728838
- Molecular Formula:
- C20H24FN3O4
- Molecular Weight:
- 389.42
- MDL Number:
- MFCD00864925
- MOL File:
- 127294-70-6.mol
- MSDS File:
- SDS
| Melting point | 137°C |
|---|---|
| Boiling point | 608.3±55.0 °C(Predicted) |
| Density | 1.40±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | H2O : < 0.1 mg/mL (insoluble)DMSO : 0.67 mg/mL (1.72 mM; Need ultrasonic) |
| pka | 6.44±0.50(Predicted) |
| BRN | 8362117 |
| CAS DataBase Reference | 127294-70-6(CAS DataBase Reference) |
| FDA UNII | Q022B63JPM |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H228 | |||||||||
| Precautionary statements | P403-P501c | |||||||||
| WGK Germany | 3 | |||||||||
| NFPA 704 |
|
Balofloxacin price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 25504 | Balofloxacin ≥98% | 127294-70-6 | 500mg | $37 | 2024-03-01 | Buy |
| Cayman Chemical | 25504 | Balofloxacin ≥98% | 127294-70-6 | 1g | $62 | 2024-03-01 | Buy |
| Cayman Chemical | 25504 | Balofloxacin ≥98% | 127294-70-6 | 5g | $266 | 2024-03-01 | Buy |
| Cayman Chemical | 25504 | Balofloxacin ≥98% | 127294-70-6 | 10g | $458 | 2024-03-01 | Buy |
| Usbiological | 264273 | Balofloxacin | 127294-70-6 | 250mg | $233 | 2021-12-16 | Buy |
Balofloxacin Chemical Properties,Uses,Production
Description
Balofloxacin, a novel orally-active fluoroquinolone antibiotic, was introduced in South Korea for the treatment of urinary tract infections (UTI). It can be synthetized by reaction of 3-(methylamino)piperidine with the classical 4-quinolone-3-carboxylic acid template. In vitro antibacterial activity of balofloxacin against gram-positive bacteria (Staphylococcus aureus including methicillin-resistant S. aureus, Staphylococcus epidermis, Streptococcus pneumonia, Streptococcus pyrogenes) was almost equal to that of sparfloxacin or tosufloxacin, in contrast its activity against gram-negative bacteria was 2 times or more lower. In clinical trials, balofloxacin was well tolerated and showed comparable efficacy to ofloxacin in patients with UTls. After oral administration, balofloxacin was well absorbed, and was primarily eliminated unchanged in the urine with an elimination half-life of approximately 8 h. In animal studies, balofloxacin did not exhibit any phototoxicity.
Chemical Properties
Crystalline Solid
Originator
Chugai Pharmaceutical (Japan)
Uses
Fluorinated quinolone antibacterial.
Uses
Balofloxacin is quinolone antibiotic, inhibiting the synthesis of bacterial DNA by interference with the enqyme DNA gyrase.
Manufacturing Process
(1) A mixture of ethyl 1-cyclopropyl-6,7,8-trifluoro-1,4-dihydro-4-
oxoquinoline-3-carboxylate (933 mg), 3-acetamidopiperidine (710 mg),
triethylamine (400 mg) and dimethylsulfoxide (10 ml) was heated at 100°C
for 2 hours with stirring. Thereafter the mixture was cooled down and ice
water was added thereto. The resulting mixture was extracted with chloroform
and the chloroform layer was washed with water three times before being
dried over anhydrous sodium sulfate. Removal of the solvent in vacuum
followed by purification by silica gel column chromatography (chloroformethanol)
gave ethyl 7-(3-acetamidopiperidin-1-yl)-1-cyclopropyl-6,8-difluoro-
1,4-dihydro-4-oxo quinoline-3-carboxylate (930 mg). Re-crystallization from
ethanol-ether afforded a colorless crystalline substance (MP: 217°-218°C).
(2) Ethyl 7-(3-acetamidopiperidin-1-yl)-1-cyclopropyl-6,8-difluoro-1,4-dihydro-
4-oxo-quinoline-3-carboxylate obtained from the foregoing step:
(a) (433 mg) of above product was dissolved in 6 N hydrochloric acid (5 ml)
and heated at 100°C. for 2.5 hours with stirring. After the removal of the
solvent in vacuum, methanol was added to the residue and the insoluble
materials were filtered off. Removal of the solvent followed by purification by
silica gel column chromatography (chloroform-methanol) gave hydrochloride
of 7-(3-aminopiperidin-1-yl)-1-cyclopropyl-6,8-difluoro-1,4-dihydro-4-
oxoquinoline-3-carboxylic acid (colorless, crystalline-powder). MP: color
change at about 272°C, decomposition at about 280°C.
(b) A mixture of 7-(3-acetamidopiperidin-1-yl)-1-cyclopropyl-6,8-difluoro-1,4-
dihydro-4-oxoquinoline-3-carboxylic acid (4.05 g), sodium methoxide (2.16 g)
and N,N-dimethylformamide (120 ml) was stirred for 2 hours at 100°-140°C.
The reaction mixture was concentrated in vacuum and water was added to the
residue. The mixture was neutralized with 1 N hydrochloric acid and the
neutralized mixture was then concentrated in vacuum. Purification of the
concentrated mixture by silica gel column chromatography (chloroformmethanol)
gave 7-(3-acetamidopiperidin-1-yl)-1-cyclopropyl-6-fluoro-1,4-
dihydro-8-methoxy-4-oxoquinoline-3-carboxylic acid. MP: 248°-250°C.
(c) 7-(3-Acetamidopiperidin-1-yl)-1-cyclopropyl-6-fluoro-1,4-dihydro-8-
methoxy-4-oxoquinoline-3-carboxylic acid (1.25 g) above obtained was
suspended in 6 N hydrochloric acid (30 ml) and ethanol (5 ml) and heated at
100°C for 3 hours. Then the reaction mixture was concentrated in vacuum
and the residue was purified by silica gel column chromatography
(chloroform:methanol:ammonium hydroxide=100:30:5) to afford 7-(3-
aminopiperidin-1-yl)-1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-4-
oxoquinoline-3-carboxylic acid. MP: 176°-177°C.
brand name
Q-Roxin
Therapeutic Function
Antibacterial
Pharmaceutical Applications
A 6-fluoro-8-methoxy quinolone derivative. It has good antistaphylococcal activity (MIC 0.4–4 mg/L), but is inactive against methicillin-resistant Staph. aureus (MRSA) and quinolone-resistant Staph. aureus; Str. pneumoniae is inhibited by 0.4 mg/L. It has good activity against Enterobacteriaceae, but is inactive against Ps. aeruginosa (MIC 8–16 mg/L). After a 200 mg oral dose a peak level of 1.7 mg/L is reached in 1 h. The apparent elimination halflife is about 8 h, rising to 13 h in elderly subjects. Plasma protein binding is about 16%. It was withdrawn from the market in Japan because of adverse events, but is available in China.
Balofloxacin Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Shaanxi Yikanglong Biotechnology Co., Ltd. | 17791478691 | yklbiotech@163.com | CHINA | 296 | 58 |
| Shanghai Arbor Chemical Co., Ltd. | 021-60451682 | act@arborchemical.com | CHINA | 906 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 | info@longyupharma.com | China | 2531 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
View Lastest Price from Balofloxacin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-03-06 | Balofloxacin
127294-70-6
|
US $1.90 / KG | 1KG | 99% | 10 ton | Hebei Guanlang Biotechnology Co,.LTD | |
 |
2021-09-29 | Balofloxacin
127294-70-6
|
US $0.00 / KG | 1KG | 98.0% | 500kg/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2021-07-20 | Balofloxacin USP/EP/BP
127294-70-6
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited |
-

- Balofloxacin
127294-70-6
- US $1.90 / KG
- 99%
- Hebei Guanlang Biotechnology Co,.LTD
-

- Balofloxacin
127294-70-6
- US $0.00 / KG
- 98.0%
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Balofloxacin USP/EP/BP
127294-70-6
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited