Isopropenylboronic acid pinacol ester
- CAS No.
- 126726-62-3
- Chemical Name:
- Isopropenylboronic acid pinacol ester
- Synonyms
- 4,4,5,5-tetramethyl-2-(prop-1-en-2-yl)-1,3,2-dioxaborolane;2-Isopropenyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;Isopropenylboronic acid picol ester;Isopropenylboronic acid pinacol este;Isopropenylboronic acid pinacol ester;2-Isopropenyl boronic acid pinacol ester;4,4,5,5-tetramethyl-2-(prop-1-en-2-yl)-1,3,2-;4,4,5,5-Tetramethyl-2-(isopropenyl)-1,3,2-dioxaborolane;4,4,5,5-Tetramethyl-2-(prop-1-en-2-yl)-1,3,3-dioxaborolane;4,4,5,5-tetramethyl-2-(1-methylethenyl)-1,3,2-dioxaborolane
- CBNumber:
- CB0972288
- Molecular Formula:
- C9H17BO2
- Molecular Weight:
- 168.04
- MDL Number:
- MFCD08276843
- MOL File:
- 126726-62-3.mol
- MSDS File:
- SDS
| Melting point | 157-161 °C |
|---|---|
| Boiling point | 47-49 °C/9 mbar |
| Density | 0.894 g/mL at 25 °C |
| refractive index | 1.4320 |
| Flash point | 42 °C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Liquid |
| color | Clear, colorless to brown |
| InChIKey | SVSUYEJKNSMKKW-UHFFFAOYSA-N |
| CAS DataBase Reference | 126726-62-3 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H226-H315-H317-H319-H335-H412 |
| Precautionary statements | P210-P233-P273-P280-P303+P361+P353-P305+P351+P338 |
| Hazard Codes | Xi |
| Risk Statements | 10-36/37/38-43 |
| Safety Statements | 16-26-36/37 |
| RIDADR | UN 1993 |
| WGK Germany | 3 |
| TSCA | No |
| HazardClass | IRRITANT |
| PackingGroup | Ⅲ |
| HS Code | 29349990 |
Isopropenylboronic acid pinacol ester price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 663212 | Isopropenylboronic acid pinacol ester contains phenothiazine as stabilizer, 95% | 126726-62-3 | 5g | $113 | 2024-03-01 | Buy |
| Sigma-Aldrich | 663212 | Isopropenylboronic acid pinacol ester contains phenothiazine as stabilizer, 95% | 126726-62-3 | 25g | $337 | 2024-03-01 | Buy |
| TRC | I821825 | Isopropenylboronic acid pinacol ester | 126726-62-3 | 25g | $395 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 35461 | 4,4,5,5-Tetramethyl-2-(prop-1-en-2-yl)-1,3,2-dioxaborolane 95+% | 126726-62-3 | 1g | $315 | 2021-12-16 | Buy |
| SynQuest Laboratories | 6H60-1-Y2 | Isopropenylboronic acid, pinacol ester 97% | 126726-62-3 | 100g | $368 | 2021-12-16 | Buy |
Isopropenylboronic acid pinacol ester Chemical Properties,Uses,Production
Description
Isopropenylboronic acid pinacol ester is a versatile ester reagent used for palladium-catalyzed Suzuki-Miyaura cross-coupling processes, inverse-electron-demand Diels-Alder reaction, Simmons-Smith cyclopropanation reaction, polyene cyclization, stereoselective aldol reactions, Grubbs cross-metathesis reaction, intramolecular Suzuki-Miyaura reaction, Stereoselective cross-metathesis, dipolar cycloaddition, iodosulfonylation, asymmetric conjugate addition and intramolecular hydroacylation and preparation of various therapeutic kinase and enzymatic inhibitors1. It can be used as an intermediate in the synthesis of variety of cyclic and acyclic organic compounds. It is also shown that the α-Substituted Allyl/Croty of this compound can be used for highly Diastereoand Enantioselective allylboration of aldehydes2.
Sources
- https://www.sigmaaldrich.com/catalog/product/aldrich/663212?lang=en®ion=US
- https://www.trc-canada.com/product-detail/?I821825
Uses
suzuki reaction
Uses
Isopropenylboronic Acid Pinacol Ester, can be used as an intermediate in the synthesis of variety of cyclic and acyclic organic compounds. It is also shown that the α-Substituted Allyl/Croty of this compound can be used for highly Diastereo- and Enantioselective allylboration of aldehydes.
Uses
Reagent used for
- Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions
- Inverse-electron-demand Diels-Alder reaction
- Simmons-Smith Cyclopropanation Reaction
- Polyene cyclization
- Stereoselective aldol reactions
- Grubbs cross-metathesis reaction
- Intramolecular Suzuki-Miyaura reaction
- Stereoselective cross-metathesis
- Dipolar cycloaddition
- Iodosulfonylation
- Asymmetric conjugate addition and intramolecular hydroacylation
Reagent used in preparation of various therapeutic kinase and enzymatic inhibitors
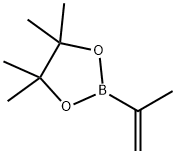
Isopropenylboronic acid pinacol ester Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Zjartschem | +86-571-8723 8903 jocelynpan@zjarts.com | jocelynpan@zjarts.com | CHINA | 993 | 58 |
| Shanghai Arbor Chemical Co., Ltd. | 021-60451682 | act@arborchemical.com | CHINA | 906 | 58 |
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 | figo.gao@foxmail.com | China | 6992 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| Wuhan Chemwish Technology Co., Ltd | 027-67849912 | sales@chemwish.com | CHINA | 10828 | 58 |
View Lastest Price from Isopropenylboronic acid pinacol ester manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2020-01-05 | Isopropenylboronic acid pinacol ester
126726-62-3
|
US $7.00 / KG | 1KG | 99% | 100KG | Career Henan Chemical Co |
-

- Isopropenylboronic acid pinacol ester
126726-62-3
- US $7.00 / KG
- 99%
- Career Henan Chemical Co





