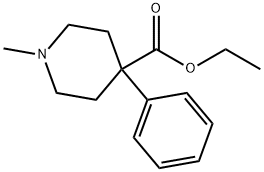
MEPERIDINE
- CAS No.
- 57-42-1
- Chemical Name:
- MEPERIDINE
- Synonyms
- Lydol;C07128;Dolsin;Demarol;Demerol;Nemerol;Petydyna;Pethidin;Pethanol;Petantin
- CBNumber:
- CB1121049
- Molecular Formula:
- C15H21NO2
- Molecular Weight:
- 247.33
- MDL Number:
- MFCD00057376
- MOL File:
- 57-42-1.mol
| Melting point | 270°C |
|---|---|
| Boiling point | 390.37°C (rough estimate) |
| Density | 1.0267 (rough estimate) |
| refractive index | 1.5130 (estimate) |
| Flash point | 11 °C |
| storage temp. | −20°C |
| pka | pKa 8.7 (Uncertain) |
| Water Solubility | 6.55g/L(25 ºC) |
| BCS Class | 1 |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | Demerol |
| FDA UNII | 9E338QE28F |
| NCI Drug Dictionary | Demerol |
| ATC code | N02AB02 |
| EPA Substance Registry System | 4-Piperidinecarboxylic acid, 1-methyl-4-phenyl-, ethyl ester (57-42-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS06,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H225-H301+H311+H331-H370 |
| Precautionary statements | P210-P260-P280-P301+P310-P311 |
| Hazard Codes | F,T |
| Risk Statements | 11-23/24/25-39/23/24/25 |
| Safety Statements | 7-16-36/37-45 |
| RIDADR | UN 1230 3/PG 2 |
| WGK Germany | 1 |
| Toxicity | A narcotic analgesic used for moderate to severe pain and during obstetrical anesthesia. Its oral LD50 in rats is 170 mg/kg. It has multiple actions qualitatively similar to those of morphine, and therapy is similar to that for morphine. Advantages over morphine concerning efficacy and reduced risk of addiction were largely illusory. This, and potential drug interactions, have resulted in a dramatic reduction in use. |
MEPERIDINE Chemical Properties,Uses,Production
Originator
Dolosal,Specia,France,1943
Uses
Analgesic (narcotic).
Definition
ChEBI: Pethidine is a piperidinecarboxylate ester that is piperidine which is substituted by a methyl group at position 1 and by phenyl and ethoxycarbonyl groups at position 4. It is an analgesic which is used for the treatment of moderate to severe pain, including postoperative pain and labour pain. It has a role as an opioid analgesic, a kappa-opioid receptor agonist, a mu-opioid receptor agonist and an antispasmodic drug. It is an ethyl ester, a piperidinecarboxylate ester and a tertiary amino compound. It is a conjugate base of a pethidine(1+).
Manufacturing Process
80 parts of finely pulverized sodium amide are added in portions each of about ? of the entire quantity, while stirring and cooling in a suitable manner, to a mixture of 756 parts of methyl-di(β-chloroethyl)-amine (prepared from di-ethanol-methylamine by means of thionyl chloride), 117 parts of benzyl cyanide and 600 parts of toluene. The reaction sets in at once at room temperature. The temperature is maintained between 30° and 40°C; when self-heating no longer occurs a further portion of the sodium amide is introduced. During the reaction heat is liberated and gaseous ammonia escapes.
The mixture is then slowly heated to the boiling point of toluene and kept
boiling for one hour under reflux. After the mixture has been allowed to cool
the sodium chloride which precipitates is separated by extraction with water.
The solution of toluene is then extracted with dilute hydrochloric acid. From
the hydrochloric acid extract the basic substance is separated in the form of
an oil by means of caustic soda solution and is introduced into ether. The
ethereal solution is dried with the aid of potassium carbonate and then
distilled.
Under a pressure of 4.5 ml the 1-methyl-4-phenyl-piperidine-4-carboxylic acid
nitrile passes over at a temperature of about 148°C in the form of a colorless
oil; under a pressure of 6 ml it passes over at about 158°C. After having been
allowed to cool the distillate solidifies completely to form a crystalline mass.
Its solidification point is at 53°C; the yield amounts to about 135 parts, that
is, about 2/3 of the theoretical yield. When recrystallized from isopropyl
alcohol the hydrochloride of the nitrile forms colorless crystals, readily soluble
in water and melting at 221° to 222°C.
The nitrile may best be saponified with methyl alcoholic potash while heating
to 190° to 200°C with application of pressure. After the methyl alcohol has
evaporated the salt is introduced into water and by the addition of dilute
mineral acid until the alkaline reaction to phenolphthalein has just
disappeared, the amphoteric 1-methyl-4-phenyl-piperidine-4-carboxylic acid is
precipitated while hot in the form of a colorless, coarsely crystalline powder.
When dried on the water bath the acid still contains 1 mol of crystal water
which is lost only at a raised temperature. The acid melts at 299°C. Reaction
with ethanol yields the ester melting at 30°C and subsequent reaction with
HCl gives the hydrochloride melting at 187° to 188°C.
brand name
Demerol (Hospira); Demerol (Sanofi Aventis).
Therapeutic Function
Narcotic analgesic
Biological Functions
Meperidine (Demerol) is a phenylpiperidine derivative
of morphine that was developed in the late 1930s as a
potential anticholinergic agent. It has some anticholinergic
side effects that lead to tachycardia, blurred vision,
and dry mouth. Meperidine is approximately onefifth
as potent as morphine and is absorbed only half as
well when administered orally as parenterally. It has a
rapid onset and short duration of action (2 hours), that
is, approximately one-fourth that of morphine.
Like morphine, meperidine has an active metabolite,
normeperidine, formed by N-demethylation of
meperidine. Normeperidine is not analgesic but is a
proconvulsant and a hallucinogenic agent. For this reason,
meperidine use in patients with renal or liver insufficiency
is contraindicated because of the decreased
clearance of the drug and its metabolite. Convulsant activity
has been documented in elderly patients given
meperidine and in patients using PCA who have decreased
renal function.
Meperidine differs from morphine in that it has far
less antitussive effect and little constipative effect. The
drug is particularly useful in cancer patients and in pulmonary
patients, in whom the cough reflex must remain
intact. However, it does have more seizure-inducing activity
than morphine. Although meperidine produces
spasms of the biliary tract and colon, such spasms are of
shorter duration than those produced by morphine.
Meperidine readily passes the placenta into the fetus.
However, respiratory depression in the newborn
has not been observed, and meperidine clearance in the
newborn is rapid in that it does not rely upon conjugation
to glucuronides. Meperidine, unlike morphine, has
not been associated with prolongation of labor; conversely,
it increases uterine contractions.
General Description
Meperidine (Demerol) was discovered in 1939 during a serendipitous screening of compounds being studied for antispasmodic activity. Mice given meperidine were noted to carry their tails in an erect position (the Straub tail reaction), which was indicative of narcotic analgesia. This led to the study of meperidine and derivatives as analgesic agents. Meperidine was found to have low potency at the receptor compared with morphine (0.2%) but much higher penetration into the brain resulting in a compound with about 10% of the potency of morphine.
Structural changes that increase the potency of meperidine include the introduction of an mhydroxyl on the phenyl ring, substituting the methyl on the N for a phenylethyl or a p-aminophenylethyl. Replacing the N-methyl with an N-allyl or N-cyclopropylmethyl group does not generate an antagonist, unlike the similar substitution of the morphine congeners. Meperidine quickly penetrates the blood-brain barrier and thus has a quick onset of activity and a high abuse potential.
Precautions
Contraindications are similar to those of morphine. In addition, because normeperidine accumulates in renal dysfunction and meperidine accumulates in hepatic dysfunction, meperidine is contraindicated in such patients because of convulsant effects. Similarly, the use of meperidine is contraindicated in patients who have a history of seizures or who are taking medication to prevent seizures. Phenytoin administered for seizures may reduce the effectiveness of meperidine by increasing the metabolism of the drug in the liver. Meperidine is not generally used in patients with cardiac dysfunction, since its anticholinergic effects can increase both heart rate and ectopic beats.
MEPERIDINE Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
57-42-1(MEPERIDINE)Related Search:
1of4