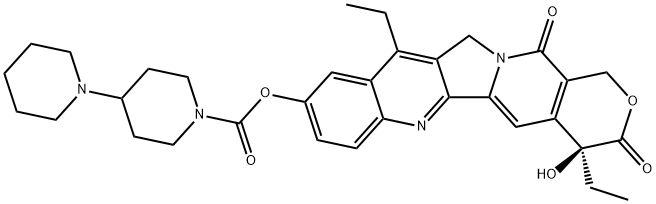
Irinotecan
- CAS No.
- 97682-44-5
- Chemical Name:
- Irinotecan
- Synonyms
- CPT-11;IRINOTECAN HCL;CAMPTOSAR;Irinotecan(TECANS);Irinotecan (free Base);[1,4'-BIPIPERIDINE]-1'-CARBOXYLIC ACID;(19S)-10,19-diethyl-19-hydroxy-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.0^{2,11}.0^{4,9}.0^{15,20}]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaen-7-yl 4-(piperidin-1-yl)piperidine-1-carboxylate;DQ-2805;SN-38-11;IRINOTECAN
- CBNumber:
- CB1124156
- Molecular Formula:
- C33H38N4O6
- Molecular Weight:
- 586.68
- MDL Number:
- MFCD01862255
- MOL File:
- 97682-44-5.mol
- MSDS File:
- SDS
| Melting point | 222-223° |
|---|---|
| Boiling point | 873.4±65.0 °C(Predicted) |
| Density | 1.40±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Acetonitrile (Slightly, Heated, Sonicated), DMSO (Slightly), Methanol (Slightly) |
| pka | 11.20±0.20(Predicted) |
| form | Solid |
| color | White to Brown |
| CAS DataBase Reference | 97682-44-5(CAS DataBase Reference) |
| FDA UNII | 7673326042 |
| NCI Dictionary of Cancer Terms | Camptosar; CPT 11 |
| NCI Drug Dictionary | Camptosar |
| ATC code | L01CE02 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS05 |
|---|---|
| Signal word | Danger |
| Hazard statements | H315-H361-H302-H318-H373 |
| Precautionary statements | P264-P270-P301+P312-P330-P501-P201-P202-P281-P308+P313-P405-P501-P260-P314-P501-P264-P280-P302+P352-P321-P332+P313-P362-P280-P305+P351+P338-P310 |
| Hazard Codes | Xn |
| Risk Statements | 22 |
| RTECS | DW1061000 |
Irinotecan price More Price(38)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Tocris | 2688 | CPT11 ≥99%(HPLC) | 97682-44-5 | 10 | $229 | 2021-12-16 | Buy |
| Tocris | 2688 | CPT11 ≥99%(HPLC) | 97682-44-5 | 50 | $960 | 2021-12-16 | Buy |
| Usbiological | 255047 | CPT 11 | 97682-44-5 | 10mg | $480 | 2021-12-16 | Buy |
| TRC | I767523 | (+)-Irinotecan | 97682-44-5 | 50mg | $60 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FI33814 | Irinotecan | 97682-44-5 | 100mg | $60 | 2021-12-16 | Buy |
Irinotecan Chemical Properties,Uses,Production
Uses
Irinotecan is a topoisomerase I inhibitor for LoVo cells and HT-29 cells with IC50 of 15.8 μM and 5.17 μM, respectively
Uses
(+)-Irinotecan is a Topo I inhibitor.
Definition
ChEBI: A member of the class of pyranoindolizinoquinolines that is the carbamate ester obtained by formal condensation of the carboxy group of [1,4'-bipiperidine]-1'-carboxylic acid with the phenolic hydroxy group of (4S)-4,11-diethyl-4,9-dihydro y-1H-pyrano[3',4':6,7]indolizino[1,2- hydrochloride]quinoline-3,14-dione. Used (in the form of its hydrochloride salt trihydrate) in combination with fluorouracil and leucovorin, for the treatment of patients with metastatic adenocarcino a of the pancreas after disease progression following gemcitabine-based therapy. It is converted via hydrolysis of the carbamate linkage to its active metabolite, SN-38, which is ~1000 times more active.
brand name
Camptosar (Pharmacia &Upjohn) .
Biological Activity
irinotecan (cpt-11), a prodrug for treating metastatic colorectal cancer, is a topoisomerase i inhibitor for lovo cells and ht-29 cells with ic50 of 15.8 μm and 5.17 μm, respectively [1].in vivo, irinotecan is converted to sn-38, its most active metabolite, by carboxylesterase converting enzyme (cce) [2].
in vitro
irinotecan induced similar amounts of cleavable complexes in lovocells and ht-29 cell lines with the ic50 of 15.8 μm and 5.17 μm, respectively [1].after addition of 157 mm irinotecan to plasma, sn-38 concentration showed linear increase during the first 60-min period, followed by a plateau.in the first 60 min, mean and standard deviation of the conversion rate were 515.9 ± 50.1 pmol/ml/h (n = 69), with a coefficient of variation of 0.097 [2]. irinotecan (cpt-11) was significantly more active in sclc than in nsclccelllines (p = 0.0036). ce activity appeared to be associated with higher sensitivity to cpt-11 in human lung cancercelllines and may partly explain the difference in the in vitro sensitivity to cpt-11 between sclc and nsclccells [3].in vitro, the sensitivity to cpt-11 and sn-38 was highest in ls174t and colo 320cells, intermediate in sw1398cellsand lowest in colo 205 and widr cells. the activity of sn-38 was 130 to 570 times than cpt-11[4].
in vivo
in colo 320 xenografts, irinotecan induced a maximum growth inhibition of 92% [4].a single dose of irinotecan significantly increased amounts of topoisomerase i covalently bound to dna in stomach, duodenum, colon and liver. concomitantly, the irinotecan-treated group exihibited significantly higher amounts of dna strand breaks in colon mucosa cells compared to the control group [5].
References
[1]. tobin p, clarke s, seale j p, et al. the in vitro metabolism of irinotecan (cpt‐11) by carboxylesterase and β‐glucuronidase in human colorectal tumours[j]. british journal of clinical pharmacology, 2006, 62(1): 122-129.
[2]. shingyoji m, takiguchi y, watanabe‐uruma r, et al. in vitro conversion of irinotecan to sn‐38 in human plasma[j]. cancer science, 2004, 95(6): 537-540.
[3]. van ark-otte j, kedde m a, van der vijgh w j, et al. determinants of cpt-11 and sn-38 activities in human lung cancer cells[j]. british journal of cancer, 1998, 77(12): 2171.
[4]. jansen w j m, zwart b, hulscher s t m, et al. cpt-11 in human colon-cancer cell lines and xenografts: characterization of cellular sensitivity determinants[j]. international journal of cancer, 1997, 70(3): 335-340.
[5]. na y s, jung k a, kim s m, et al. the histone deacetylase inhibitor pxd101 increases the efficacy of irinotecan in in vitro and in vivo colon cancer models[j]. cancer chemotherapy and pharmacology, 2011, 68(2): 389-398.
Irinotecan Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hangzhou ICH Biofarm Co., Ltd | +undefined8613073685410 | sales@ichemie.com | China | 985 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9350 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 967 | 58 |
Related articles
- Medical uses, Mechanism and Toxicities of Irinoteca
- Irinotecan, sold under the brand name Camptosar among others, is an antineoplastic enzyme inhibitor primarily used in the trea....
- Nov 14,2019
View Lastest Price from Irinotecan manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-12 | lrinotecan
97682-44-5
|
US $0.00 / kg | 1kg | 99% | 2000ton | Shaanxi Haibo Biotechnology Co., Ltd | |
 |
2023-11-01 | Irinotecan
97682-44-5
|
US $0.00-0.00 / kg | 1kg | 99.9% | 20 tons | Hangzhou ICH Biofarm Co., Ltd | |
 |
2023-06-15 | Irinotecan
97682-44-5
|
US $825.00 / g/Bag | 10g | 99% | 1kg | Baoji Guokang Bio-Technology Co., Ltd. |
-

- lrinotecan
97682-44-5
- US $0.00 / kg
- 99%
- Shaanxi Haibo Biotechnology Co., Ltd
-

- Irinotecan
97682-44-5
- US $0.00-0.00 / kg
- 99.9%
- Hangzhou ICH Biofarm Co., Ltd
-

- Irinotecan
97682-44-5
- US $825.00 / g/Bag
- 99%
- Baoji Guokang Bio-Technology Co., Ltd.
97682-44-5(Irinotecan)Related Search:
1of4