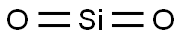Silica glass
- CAS No.
- 60676-86-0
- Chemical Name:
- Silica glass
- Synonyms
- KIESELGUHR;SILICONE DIOXIDE;CELITE 545;HYFLO;MICROSILICA;fused;Fused silica;KIESELGEL;KIESELGUR;FILTER AGENT
- CBNumber:
- CB1199394
- Molecular Formula:
- O2Si
- Molecular Weight:
- 60.0843
- MDL Number:
- MFCD01778719
- MOL File:
- 60676-86-0.mol
| Melting point | 1610 °C(lit.) |
|---|---|
| Boiling point | 2950°C |
| Density | 2.6 g/mL at 25 °C(lit.) |
| refractive index |
n |
| solubility | insoluble in H2O, acid solutions; soluble in HF |
| form | rod (1/8") |
| CAS DataBase Reference | 60676-86-0 |
| NIST Chemistry Reference | Silicon dioxide(60676-86-0) |
| EPA Substance Registry System | Silica vitreous (60676-86-0) |
| Modulus of Elasticity | 70.0 - 78.0 GPa |
|---|---|
| Poissons Ratio | 0.17 |
| Shear Modulus | 31.0 GPa |
| Hardness, Mohs | 5.5 - 7.0 |
| Hardness, Knoop | 820 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H335-H373 |
| Precautionary statements | P305+P351+P338-P314 |
| Hazard Codes | T,Xn |
| Risk Statements | 48/20-36/38-45-36/37 |
| Safety Statements | 22-24/25-45-26-53-36 |
| WGK Germany | 2 |
| RTECS | VV7311000 |
| HS Code | 28112200 |
| Toxicity | LDLo intravenous in cat: 5mg/kg |
Silica glass price More Price(30)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | CX0574 | Celite® 545 | 60676-86-0 | 500g | $50.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | CX0574 | Celite® 545 | 60676-86-0 | 2.5kg | $92.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | CX0574 | Celite® 545 | 60676-86-0 | 12kg | $357 | 2024-03-01 | Buy |
| Sigma-Aldrich | BR74511 | BRAND® filter funnel support with base plate for 4 funnels | 1EA | $259 | 2024-03-01 | Buy | |
| Sigma-Aldrich | 914150 | Silica dioxide- sorbent milled nanofiber | 60676-86-0 | 5G | $225 | 2024-03-01 | Buy |
Silica glass Chemical Properties,Uses,Production
Overview
Quartz glass, Fused quartz, and silica glass are synonyms for glass made from high purity quartz. Quartz Glass is manufactured by melting naturally occurring high purity quartz sand at approximately 2000 °C, using either an electrically heated furnace (electrically fused) or a gas/oxygen-fueled furnace (flame fused). Fused quartz is normally transparent.
Quartz glass is a kind of special glass composed of a single component of silica. According to its purity, it can be divided into high-purity quartz glass, ordinary quartz glass and doped quartz glass, three categories. According to transparency, it is divided into transparent and opaque, two categories; because of a series of excellent characteristics such as high purity, excellent performance for light penetration, high temperature resistance capability, thermal shock resistance, stable chemical property and resistance to radiation as well as electrical insulation, it is known as the "king of glass"; it can be made of tubes, rods, plates, blocks and fibers, can be processed into various shapes of equipment containers, can also be cut, polished, polished into prisms, lenses and other optical components. Being mixed with a small amount of impurities can be made of new varieties of special properties such as ultra-low temperature expansion, fluorescent quartz glass, etc.; thus widely used in semiconductor, new light source, optical, instrumentation, thermal, metallurgical, chemical and building materials industry and laser technology, space technology, astronomy, nuclear engineering, optical communication and other high-tech fields.
A number of unique optical, mechanical and thermal properties have made quartz glass an indispensable material in the fabrication of high-tech products.
Physical and Chemical Properties
It has high mechanical strength, high temperature resistance, and low coefficient of thermal expansion, excellent thermal shock resistance, good chemical stability, high dielectric strength and small refractive index. It has excellent property for being penetrated by light at the range of ultraviolet, visible and infrared light regions. It has excellent low or high temperature insulation performance;
An overview of the quartz glass, physical and chemical properties, preparation methods, applications, etc. are edited by Ding Hong from Chemicalbook. (2015-11-16)
Application
- Transparent quartz glass is mainly used for semiconductor, electric light source, metallurgy and chemical industry and national defense science and technology.
- Opaque quartz glass is mainly used for the chemical industry synthesis reactor, acid-resistant pipes, phosphor, optical glass melting pot, glass furnace refractories and other electrical and thermal materials.
- Optical quartz glass UV optical quartz glass is mainly used for precision optical instruments, analytical instruments, astronomic instruments and space technology. Infrared optical quartz glass is mainly used for infrared detection and tracking system, apparatus of the precision optical instrument, the observation lens of the industrial furnace, missile radome, radar delay line and color TV delay line chip and so on.
- Low-expansion quartz glass as a cavity material can improve the measurement accuracy of He-Ne laser and timing accuracy of atomic clock; as a kind of precision optical components, it is a high quality material for the lightweight astronomical telescope as well as a good material of the hagioscope for the spacecraft.
- Fold-resistant quartz glass is used as materials for the anti-thermal shock optical window and structural material, especially used at high temperatures, which catalyze the crystallization of quartz glass with water vapor and oxygen.
- Alkali-resistant quartz glass can be used for the production of metal halide lamp. It has stable light color, high light maintenance rate so that life expectancy can be doubled and the life of the self-ballasted fluorescent high-pressure mercury lamp can be prolonged by more than three times, being a kind of ideal material for the manufacturing of the new light source system. It is mainly applied to various kinds of high-intensity gas discharge lamps of third-generation electric light source such as anti-sodium lamp, sodium ingot holding lamps, iodine filing lamp and high-pressure mercury lamp.
- Doped quartz glass can be used in fields of laser range finder, laser fusion and diamond laser drilling.
- UV filter quartz glass: UV filter quartz glass is suitable for the manufacturing of high-pressure mercury lamp, germicidal lamp and neon lamp to be applied to medical, film and other daily electric lighting field, preventing the radiation of short-wave ultraviolet on the human body and the formation of ozone in the air. Long wavelength ultraviolet light quartz glass, used as the platemaking light source of the colored photography, is conducive to the improvement of color chromaticity, used as the third generation of solid-state lasers and filter materials. The laser efficiency can be increased by 20 to 50% and the lifespan of the light source can be extended to more than a thousand times. It can be also eliminated of the damage of UV radiation on the human body and the corrosion of filter on the device and environmental pollution, allowing the laser miniaturization.
- Quartz glass brick is mainly used as the refractory materials for making melting low-alkali glass furnace.
- Quartz glass-ceramic used in metallurgy, chemical industry, glass, national defense and scientific research and other industries, especially for precision casting, infiltration nozzle of continuous casting steel and the fluid gate of the float glass melting furnace.
- Fluorescence quartz glass is suitable for ultraviolet fluorescence instrument, high pressure liquid chromatography and other instruments.
- Quartz glass fiber: the laser medical fiber made of quartz fiber can transport the laser into the cavity of the human body to be combined with other drugs. This can treat the cancers of the esophagus, trachea, rectum, stomach and other cavity as well as cancer of body surface such as breast and epidermis. The optical fiber sensor made of quartz fiber can transmit temperature, pressure, displacement, speed, voltage, and current as well as solution concentration. Compared with the traditional sensing technology, it is simple, anti-electromagnetic interference, fast and sensitive. It is small and exquisite when made of ultraviolet radiation meter to be used for UV radiative detection in the medical, chemical, electronics and public security. It has high sensitivity, good effect and low price.
- Quartz glass spring is used in thermobalance, gravimeter, seismograph and other precision instruments. It is suitable for vacuum deposition.
Preparation
(1) Chemical Vapor Deposition method (CVD)
The silicon tetrachloride liquid is brought into the burner by the gas bubbling method, then the high temperature flame is produced by the combustion of the hydrogen-oxygen gas to hydrolyze and melt the gaseous silicon tetrachloride. After the reaction, the silicon dioxide is deposited on the substrate on. CVD deposition device can be divided into two kinds, horizontal and vertical according to the structure. In contrast, the base rod applied vertical layout inside the vertical deposition device, generating a higher deposition rate, being able to prepare large-size quartz glass.
The reaction is as follows:
SiCl4 (gas) + 2H2 + O2 (gas) → SiO2 ↓ + 4HCl ↑
Quartz glass synthesized by CVD method has low metal impurity content, and its performance is better than that of fused silica glass, such as high deep ultraviolet transmittance, high optical uniformity and excellent radiation resistance, which can meet the needs of some high-tech fields. However, the quartz glass prepared by the CVD method has a serious drawback: since the preparation process is always in a hydrogen excess atmosphere, the content of hydroxyl groups in the prepared quartz glass is high. The researchers generally believe that the hydroxyl group led to that infrared absorption spectrum of quartz glass has a strong absorption peak at 2.73 μm, and the presence of hydroxyl groups can also lead to lower density of quartz glass, thereby reducing its mechanical properties and thermal properties.
(2) High-frequency plasma flame method
The most relevant part to the synthesis process is the plasma torch part in the whole deposition device. The torch is four-tube (shown in Fig. 3): the innermost quartz glass tube is used for conveying raw material gas, the second quartz glass tube is used for conveying oxygen, The third layer of quartz glass tube is used for conveying the working gas, the outermost layer adopts the oxygen-containing gas as the cooling gas, and the high-frequency coil surrounds the outermost quartz glass tube to excite the plasma; the raw material gas transported by the inner tube, after being mixed with the oxygen transported by the second layer, enters into the plasma area for reaction, and then generate quartz glass particles to be deposited on the base for completing the process of vitrification and obtaining high-quality hydrogen-free quartz glass ingot or quartz glass mound.
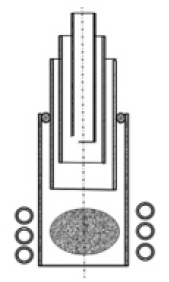
Figure 1 is the torch of four-tube quartz
References
https://www.heraeus.com/en/hqs/fused_silica_quartz_knowledge_base/properties/properties.aspx
http://www.thequartzcorp.com/en/applications/quartz-glass.html
Chemical Properties
Made up of spherical submicroscopic particles under 0.1 micron in size.
Chemical Properties
Amorphous silica, the noncrystalline form of SiO2, is a transparent to gray, odorless, amorphous powder
Occurrence
This material is known largely as a synthetic material, but there are instances of the material occurring in nature. Vitreous tubes called fulgurites are produced when lightning fuses quartz sand. Large deposits of fulgurite exist in the Libyan desert. Vitreous silica can also be produced by meteor impact. The impact leads to rapid adiabatic heating of the quartz above its melting point. The quartz forms a glass on cooling. Examples of this type of vitreous silica have been found near Canyon Diablo, Arizona, and in meteorite craters in Australia and Arabia.
Uses
Suitable for vacuum deposition.
Uses
Chemical Applications. Because of its excellent chemical durability, high purity, thermal shock resistance, and usefulness at high temperature, vitreous silica has a wide range of applications in chemical analysis and preparations. Tubing, rods, crucibles, dishes, boats, and other containers and special apparatus are available in both transparent and nontransparent varieties. Because of its inertness, vitreous silica is used as a chromatographic substrate in the form of microparticles, capillary tubing, and open columns for high resolution gas chromatography.
Thermal Applications. The protection of precious-metal thermocouples in high temperature pyrometry is an important application of vitreous silica. Although satin tubing is usually employed, transparent tubes are superior for protecting couples when used in a reducing atmosphere.
Optical Applications. Vitreous silica is ideal for many optical applications because of its excellent uv transmission, resistance to radiation darkening, optical polishing properties, and physical and chemical stability. It is used for prisms, lenses, cells, windows, and other optical components where uv transmission is critical. Cuvettes used in scatter and spectrophotometer cells are manufactured from fused silica and fused quartz because of the transmissive properties and high purity.
Mechanical Applications. The volume of vitreous silica used for fibers is a very small part of the total consumption. However, some interesting and significant applications have been developed in the laboratory, particularly in the area of measurements.
Electronic Applications. In electronic systems, such as radar and computers, signal delay is sometimes necessary. A transducer converts electrical signals to ultrasonic elastic waves, which pass through a connecting medium to another transducer, where the waves are reconverted to electrical signals.
Space and Astronomy. Vitreous silica is used in several space-based applications because of static fatigue (slow crack growth), thermal stability, and radiation resistance. Every U.S. space vehicle having service personnel, including Mercury, Gemini, Apollo, and space shuttle vehicles, has been equipped with windows made of high optical-quality vitreous silica (Corning Code 7940 or 7980) in order to have the clarity needed for visual, photographic, and television-based observations. The space shuttle utilizes triple-layer windows that have outer and central panes of vitreous silica with a tempered aluminosilicate inner pane. The outer pane is thinner for thermal endurance, whereas the two inner panes are thicker to supply strength.
Uses
Concrete, grouts, mortars, elastomers, refrac- tory and coating applications.
Definition
ChEBI: Silicon dioxide is a silicon oxide made up of linear triatomic molecules in which a silicon atom is covalently bonded to two oxygens.
Production Methods
Modern manufacturing processes of vitreous typically involve the fusion or viscous sintering of silica particles; the particles can be derived from sand crystals or are produced through a chemical process, e.g., flame hydrolysis or sol–gel. In one practice of the flame hydrolysis process, the powder is produced and fused into glass a single step, without the isolation of a porous body. Dopant and additive profiles are concentration are then controlled by the deposition conditions. When a process involving a discrete porous silica body as an intermediate is used, subsequent processing steps can be used to control dopant levels and in particular, the hydroxyl level of the final glass. The choice of fabrication method is often dictated by the end-use specifications. Flame hydrolysis or similar chemical techniques that allow for the production of very high purity glass are the methods of choice for optical applications but may be economically wasteful for less demanding applications.
Translucent Vitreous Silica. Translucent vitreous silica is produced by fusion of high purity quartz sand crystals. Sand is packed around a graphite rod through which a current is passed. The resistance heating produces a plastic mass that can be blown into molds, drawn into tubing, or shaped by rolling or pressing. Separation from the graphite rod is facilitated by gaseous products formed by interfacial reaction. Because the outside is sandy, the product is known as sand-surface ware. A matte finish is obtained by mechanical buffing. A glazed surface is produced by fusing the outside surface with an electric carbon arc or flame.
Transparent Vitreous Silica. Clear, transparent, bubble-free vitreous silica may be obtained by melting natural quartz minerals by flame or plasma vapor deposition (synthetic fused silicas), and by sol–gel processing.
General Description
Silicon dioxide is one of the important constituents of sedimentary rock bauxite, basalt fibers and ceramic fibers. It is added to cement for improving the hydraulic properties of cement.
Hazard
Questionable carcinogen.
Safety Profile
An inhalation hazard. Questionable carcinogen with experimental tumorigenic data. Poison by intraperitoneal, intravenous, and intratracheal routes. See also other shca entries.
Potential Exposure
Amorphous fumed silica is used as a mineral, natural or synthetic fiber. A potential danger to those involved in the production and handling of fumed silica for paint pigments or catalysts. Diatomaceous earth is used in clarifying liquids, in manufacture of fire brick and heat insulators; used as a filtering agent; as a filler in construction materials; pesticides, paints, and varnishes. A potential danger to those involved in mining of diatomaceous earth or fabrication of products there from.
Purification Methods
Purification of silica for high technology applications uses isopiestic vapour distillation from concentrated volatile acids and is absorbed in high purity water. The impurities remain behind. Preliminary cleaning to remove surface contaminants uses dip etching in HF or a mixture of HCl, H2O2 and deionised water [Phelan & Powell Analyst 109 1299 1984].
Incompatibilities
Silica, amorphous is a noncombustible solid. Generally unreactive chemically. Incompatible with fluorine, oxygen difluoride, chlorine trifluoride. Soluble in molten alkalis and reacts with most metallic oxides at high temperature.
Waste Disposal
Sanitary landfill.
Silica glass Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 | admin01@hsnm.com.cn | China | 2118 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| HONG KONG IPURE BIOLOGY CO.,LIMITED | 86 18062405514 18062405514 | ada@ipurechemical.com | CHINA | 3465 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
| Henan Alfa Chemical Co., Ltd | +8618339805032 | alfa4@alfachem.cn | China | 12755 | 58 |
| Alfa Chemistry | +1-5166625404 | Info@alfa-chemistry.com | United States | 21317 | 58 |
Related articles
- Vitreous or Amorphous Silica
- High-purity amorphous or fused silica, also called vitreous silica, when optically translucent is a high-performance ceramic o....
- Jan 9,2024
View Lastest Price from Silica glass manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-14 | Silica glass
60676-86-0
|
US $0.00 / kg | 1kg | 99.99% | 20tons | Wuhan Han Sheng New Material Technology Co.,Ltd | |
 |
2019-12-23 | Silica glass
60676-86-0
|
US $2.00 / KG | 1KG | ≥98% | 100kg | Career Henan Chemical Co |
-

- Silica glass
60676-86-0
- US $0.00 / kg
- 99.99%
- Wuhan Han Sheng New Material Technology Co.,Ltd
-

- Silica glass
60676-86-0
- US $2.00 / KG
- ≥98%
- Career Henan Chemical Co