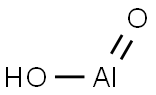Boehmite
- CAS No.
- 1318-23-6
- Chemical Name:
- Boehmite
- Synonyms
- bohmite;BOEHMITE;Nanobumite;Nanoboehmite;Boehmite (Al(OH)O);Boehmite Nanopowder;ALPHA-ALUMINA MONOHYDRATE;Aluminum Oxide(Boehmite) NanowiresA4;Aluminum OxideNanowire Dispersion A4;Aluminium Oxide (Boehmite) Nanodispersion (50nm), 20% in water
- CBNumber:
- CB1212426
- Molecular Formula:
- AlHO2
- Molecular Weight:
- 59.99
- MDL Number:
- MFCD00278830
- MOL File:
- 1318-23-6.mol
- MSDS File:
- SDS
| Density | 3.070 |
|---|---|
| solubility | insoluble in H2O; soluble in hot acid solutions,alkaline solutions |
| color | white orthorhombic crystals, crystalline |
| Water Solubility | insoluble H2O; soluble hot acids, hot alkalies [CRC10] |
| CAS DataBase Reference | 1318-23-6 |
| FDA UNII | NZO8Q0FP2E |
| EPA Substance Registry System | Boehmite (Al(OH)O) (1318-23-6) |
Boehmite Chemical Properties,Uses,Production
Description
Boehmite (γ-AlOOH), one of the two polymorphs of aluminum oxyhydroxide (the other one is diaspore, α-AlOOH), is a material useful in several commercial applications, for example, ceramics, abrasive materials, fire-retardants, adsorbents, catalysts and fillers for polymeric composites. It can also be used as raw material for producing transition aluminas (mainly γ -Al2 O3 ) with a large range of applications in catalysis and adsorption, and α-Al2 O3 for the production of ceramic materials (used in a broad range of applications, for instance mechanical parts, refractory materials and electric insulators) .
Synthesis and Characterization of Boehmites Obtained from Gibbsite in Presence of Different Environments
Chemical Properties
white; ortho-rhomb; a=0.286 nm, b=1.2227nm, c=0.380nm; hardness is 3.5–4 Mohs; obtained by hydrothermal reaction of hydroxide slurries at 200°C–250°C [KIR78] [ROB67]; solubility data are in [BOU93] and [PHI93]
Boehmite is an aluminium oxide hydroxide (γ-AlO(OH)) mineral, a component of the aluminium ore bauxite. It belongs to the orthorhombic system and has a layered structure. In each single structural layer, oxygen ions are arranged in cubic close-packed at the vertices of the octahedron, and aluminum ions are located in the center of the octahedron to form a double-layer structure. , the hydroxide radicals are located on the surface of the layered structure, and the layers are connected by hydrogen bonds.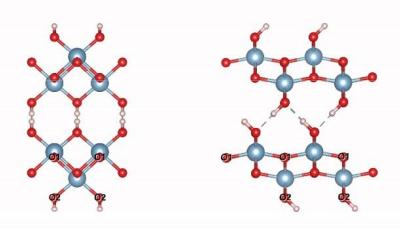
Crystal structure of boehmite
Physical properties
The most important aluminous minerals in bauxite are gibbsite (Al(OH)3), boehmite (AlO(OH)) and diaspore (AlO(OH)), however gibbsite requires lower digestion temperature to be dissolved (typically ~145oC) as compared to boehmite and diaspore (typically >240 oC), Equation (1 – 2). Accordingly, alumina refineries are usually designed to specifically process one type of bauxite. While boehmitic bauxites are usually processed using ‘high temperature’ digestion conditions (to recover boehmite) in theory a boehmitic bauxite could be processed in a low temperature plant with no or minimal process impact if the boehmite levels were sufficiently low, even though this boehmite content is not dissolved, increasing residue amount to clarification.
Al(OH)3 (s) + NaOH (aq) <=>NaAl(OH)4 (aq) (1)
AlOOH (s) + H2O (l) + NaOH (aq) <=> NaAl(OH)4 (aq) (2)
Uses
Boehmite is a high-temperature resistant aluminum oxide-hydroxide. The functional filler is used as a carrier material for catalytic converters or as a flame retardant in printed circuit boards. In the growing market of electromobility, boehmite is also finding increasing use.
the minerals boehmite (aluminum oxyhydroxide, γ-AlOOH) and gibbsite (aluminum hydroxide, α-Al(OH)3) are abundant natural ores of aluminum, as well as being important raw materials in industrial applications as adsorbents, fire retardants , coatings , catalysts, polishing agents, fillers, and fuel cells. They are important precursors for the synthesis of different alumina products, such as γ-/δ-Al2O3, chiAl2O3, and α-Al2O3, which are widely used in specialized industries including filler, catalysis, glass, ceramics, purification, paint, coating, and metallurgy. Boehmite and gibbsite are also used in sensitive or specialized applications. For example, gibbsite is used as a substrate for treatment of stomach diseases and also serve as a vaccine adjuvant, and boehmite is used as a host material for the light-emitting diodes (LEDs) .
Definition
boehmite: A mineral form of amixed aluminium oxide and hydroxide,AlO.OH. It is named after theGerman scientist J. B?hm.
Boehmite Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Zhengzhou Anhuida Chemical Co., Ltd | +8615903659408 | admin@ahdchem.com | China | 298 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Richest Group Ltd | 18017061086 | oled@richest-group.com | CHINA | 5601 | 58 |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | sale04@alfachem.cn | China | 12468 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503259 +86-19930503259 | cherry@crovellbio.com | China | 18457 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43348 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33349 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |