Strontium carbonate
- CAS No.
- 1633-05-2
- Chemical Name:
- Strontium carbonate
- Synonyms
- STRONTIUM CARBONATE POWDER;SrCO;ci77837;Nsc112224;strontianite;Strontiumcarbonat;Sulfuryl caprylate;StrontuM carbonate;STRONTIUM CARBONAE;StorntiumCarbonate
- CBNumber:
- CB1237917
- Molecular Formula:
- CO3Sr
- Molecular Weight:
- 147.63
- MDL Number:
- MFCD00011250
- MOL File:
- 1633-05-2.mol
- MSDS File:
- SDS
| Melting point | 1494 °C (lit.) |
|---|---|
| Density | 3.7 g/mL at 25 °C (lit.) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | dilute aqueous acid: slightly soluble(lit.) |
| form | Powder |
| Specific Gravity | 3.7 |
| color | white |
| Water Solubility | Soluble in ammonium chloride. Slightly soluble in ammonia and water. |
| Merck | 14,8838 |
| Solubility Product Constant (Ksp) | pKsp: 9.25 |
| Stability | Stable. Incompatible with strong acids. |
| InChIKey | LEDMRZGFZIAGGB-UHFFFAOYSA-L |
| LogP | -0.809 (est) |
| CAS DataBase Reference | 1633-05-2(CAS DataBase Reference) |
| FDA UNII | 41YPU4MMCA |
| EPA Substance Registry System | Carbonic acid, strontium salt (1:1) (1633-05-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P280-P302+P352-P304+P340 | |||||||||
| WGK Germany | - | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2836920000 | |||||||||
| NFPA 704 |
|
Strontium carbonate price More Price(70)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | NIST987 | Strontium carbonate NIST? SRM? 987, isotopic standard | 1633-05-2 | 1g | $863 | 2024-03-01 | Buy |
| Sigma-Aldrich | 204455 | Strontium carbonate 99.995% trace metals basis | 1633-05-2 | 25g | $176 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.07861 | Strontium carbonate Technipur? | 1633-05-2 | 500G | $180 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.07861 | Strontium carbonate Technipur? | 1633-05-2 | 50KG | $8750 | 2024-03-01 | Buy |
| Sigma-Aldrich | 204455 | Strontium carbonate 99.995% trace metals basis | 1633-05-2 | 5g | $59.4 | 2023-06-20 | Buy |
Strontium carbonate Chemical Properties,Uses,Production
Description
Strontium carbonate (SrCO3)(1633-05-2) belongs to the carbonate salt of strontium, which is found in nature as the mineral strontianite. It can be applied in a variety of industries. At present, strontium carbonates are commonly being applied as an inexpensive colorant in pyrotechnics since strontium and its salts produce a crimson read flame. Strontium carbonate, in general, is preferred in fireworks, compared with other strontium salts due to its inexpensive cost, nonhygroscopic property, and ability to neutralize acid. It can also be used as road flares and for preparing iridescent glass, luminous paints, strontium oxide or strontium salts and in refining sugar and certain drugs. It is also recommended as a substitute for barium to produce matte glazes. Besides, its applications involves in ceramics industry, where it serves as an ingredient in glazes, and in electric products, where it is used for the production of strontium ferrites to produce permanent magnets for loudspeakers and door magnets. Strontium carbonate is also used for manufacturing some superconductors such as BSCCO and also for electroluminescent materials.
Production Methods
Strontium carbonate occurs in nature as strontianite and can be mined from its deposit. It is, however, usually made from the mineral celestite. Celestite is fused with sodium carbonate at elevated temperatures or boiled with a solution of ammonium carbonate:
SrSO4 + Na2CO3 → SrCO3 + Na2SO4
SrSO4 + (NH4)2CO3 → SrCO3 + 2NH3 + CO2 + H2O
Strontium carbonate is insoluble in water. It precipitates from the product mixture in the second reaction. If fused with sodium carbonate, the product mixture is leached with water. Insoluble carbonate separates from the watersoluble sodium sulfate.
References
https://en.wikipedia.org/wiki/Strontium_carbonate
http://www.nanopartikel.info/en/nanoinfo/materials/strontium-carbonate
Description
Strontium carbonate has the formula of SrCO3 and the molecular weight of 147.6326 g/mol. Strontium carbonate occurs in nature as the mineral “strontianite”. The name strontianite comes from a famous location for the mineral, Strontian, Scotland. Strontianite is strontium carbonate as found naturally. It occurs as white or slightly gray orthorhombic crystals with a refractive index of 1.518. The unit-cell parameters are: a = 5.107 ? , b = 8.414 ? , c = 6.029 ? , Z = 4; V = 259.07 ? 3, Den(Calc) = 3.78. The crystal system is orthorhombic with space group Pmcn and point group 2/m, 2/m, 2/m. Strontium carbonate has only one stable form (aragonite-type structure) and temperature of precipitation has no effect on crystal form, unlike that of calcium or magnesium carbonates.
Chemical Properties
Heating strontium carbonate up from 1000°C to 1300°C causes the compound to undergo a decomposition reaction which results in the capture of thermal energy, shown here as the peak in the black curve occurring between Time = 20 and Time = 40 minutes. Cooling the system back down to 1000°C leads to reconstitution of the starting material in a carbonation reaction thereby releasing the stored thermal energy, a process shown as the valley in the black curve between Time = 50 and Time = 80 minutes.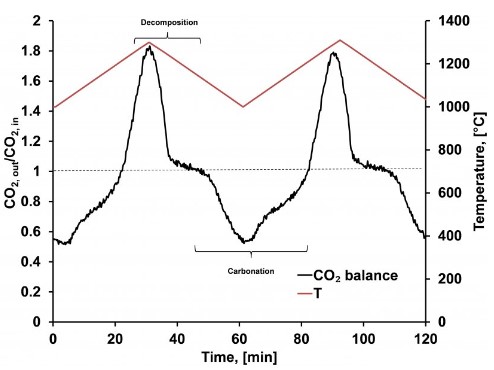
Chemical Properties
Strontium carbonate is a milky white free flowing powder. It is little more insoluble (Ksol=10-8.8) than calcium carbonate (Ksol=10-8.07), so it should not be surprising that under appropriate conditions Sr2+ can be precipitated by biogenic carbonate.
Physical properties
White orthorhombic crystals; refractive index 1.518; hygroscopic; hardness 3.5 Mohs; density 3.5 g/cm3; insoluble in water; soluble in dilute acids with liberation of carbon dioxide.
Occurrence
Strontium carbonate occurs in nature as mineral strontianite. The compound is used in pyrotechnics and ceramic ferrites. It also is used in making iridescent glass for color television tubes. Other uses are in refining sugar and preparing other strontium salts.
Uses
Used for electronic applications. It is used for manufacturing CTV to absorb electrons resulting from the cathode
Uses
The compound, SrCO3, is used in pyrotechnics and ceramic ferrites. It is also used in making iridescent glass for color television tubes. Other uses are in refining sugar and preparing other strontium salts. The most common use is as an inexpensive fireworks colorant. Strontium and its salts emit a brilliant red color in flame. Its ability to neutralize acid is also very helpful in pyrotechnics. Another similar application is in road flares. Strontium carbonate is used for electronic applications. It is used for manufacturing glass colortelevision tubes to absorb X-rays resulting from the bombardment of the cathode rays on the glass enclosure of the cathode-ray gun. SrCO3 is used in the preparation of iridescent glass, strontium oxide or strontium salts and in refining sugar.It is widely used in the ceramics industry as an ingredient in glazes. It acts as a flux and also modifies the color of certain metallic oxides. It is also used in the manufacturing of strontium ferrites for permanent magnets that are used in loudspeakers and door-magnets. Strontium carbonate can be used to produce many different strontium compounds by simply dissolving it in the corresponding acid. Strontium bicarbonate has not been isolated.
Uses
Used in the preparation of?iridescent glass, luminous paints, strontium oxide or strontium salts and in refining sugar and certain drugs
Uses
Strontium carbonate (SrCO3) is used to make radiation-resistant glass and TV picture tubes, as well as pyrotechnics.
Definition
strontium carbonate: A whitesolid, SrCO3; orthorhombic; r.d. 3.7;decomposes at 1340°C. It occurs naturallyas the mineral strontianite andis prepared industrially by boiling celestine(strontium sulphate) with ammoniumcarbonate. It can also beprepared by passing carbon dioxideover strontium oxide or hydroxide orby passing the gas through a solutionof strontium salt. It is a phosphor,used to coat the glass of cathode-rayscreens, and is also used in the refiningof sugar, as a slagging agent incertain metal furnaces, and to providea red flame in fireworks.
Preparation
Strontium carbonate occurs in nature as strontianite and can be mined from its deposit. It is, however, usually made commercially from the mineral “celestite”. Celestite is fused with sodium carbonate at elevated temperatures or boiled with a solution of ammonium carbonate.Strontium carbonate is insoluble in water. It precipitates from the product mixture in the second reaction. If fused with sodium carbonate, the product mixture is leached with water. Insoluble carbonate separates from the water-soluble sodium sulfate.
Production Methods
Strontium carbonate, formed (1) by reaction of strontium salt solution and sodium carbonate or bicarbonate solution, (2) by reaction of strontium hydroxide solution and CO2. Strontium carbonate decomposes at 1,200 °C (2,192 °F) to form strontium oxide and CO2, and is dissolved by excess CO2, forming strontium bicarbonate, Sr(HCO3)2, solution.
Definition
strontianite: A mineral form ofstrontium carbonate, SrCO3.
General Description
Strontium carbonate is insoluble in water. It is used predominantly in producing other strontium salts.
General Description
Strontianite is a strontium carbonate mineral (SrCO3). It is the original and principal source of strontium. It often occurs in white masses of radiating fibres, although pale green, yellow, or gray colours are also known. Strontianite forms soft, brittle crystals that are commonly associated with barite, celestine, and calcite in low-temperature veins. Strontianite is used in pyrotechnics to impart a red colour and in sugar refining as a clarifying agent.
Strontium carbonate Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Dalian Richfortune Chemicals Co., Ltd | 0411-84820922 8613904096939 | sales@richfortunechem.com | China | 303 | 57 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| QINGDAO HONG JIN CHEMCIAL CO.,LTD. | 532-83657313 | hjt@hong-jin.com | China | 228 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
View Lastest Price from Strontium carbonate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-11-04 | Strontium carbonate
1633-05-2
|
US $0.00-0.00 / kg | 1kg | 99% | 50000kg | Hebei Yibangte Import and Export Co. , Ltd. | |
 |
2023-09-06 | Strontium carbonate
1633-05-2
|
US $100.00 / kgkg | 1kgkg | 0.99 | 20tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-09-06 | Strontium carbonate
1633-05-2
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. |
-

- Strontium carbonate
1633-05-2
- US $0.00-0.00 / kg
- 99%
- Hebei Yibangte Import and Export Co. , Ltd.
-

- Strontium carbonate
1633-05-2
- US $100.00 / kgkg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- Strontium carbonate
1633-05-2
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
1633-05-2(Strontium carbonate )Related Search:
1of4