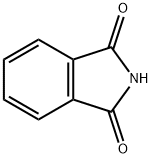
Phthalimide
- CAS No.
- 85-41-6
- Chemical Name:
- Phthalimide
- Synonyms
- Isoindoline-1,3-dione;PHTALIMIDE;ISOINDOLE-1,3-DIONE;Phthalimid;o-Phthalimide;PHTHALIC ACID IMIDE;2H-Isoindole-1,3-dione;1H-ISOINDOLE-1,3(2H)-DIONE;Benzoimide;Ftalimmide
- CBNumber:
- CB1280074
- Molecular Formula:
- C8H5NO2
- Molecular Weight:
- 147.13
- MDL Number:
- MFCD00005881
- MOL File:
- 85-41-6.mol
- MSDS File:
- SDS
| Melting point | 232-235 °C(lit.) |
|---|---|
| Boiling point | 366 °C |
| Density | 1.21 |
| vapor pressure | 0.001Pa at 25℃ |
| refractive index | 1.4700 (estimate) |
| Flash point | 165 °C |
| storage temp. | Store below +30°C. |
| solubility | water: slightly soluble(lit.) |
| pka | 8.3(at 25℃) |
| form | Crystalline Flakes |
| color | White to slightly yellow |
| PH | 3.8 (0.6g/l, H2O) |
| Water Solubility | <0.1 g/100 mL at 19.5 ºC |
| Sublimation | 366 ºC |
| Merck | 14,7373 |
| BRN | 118522 |
| InChIKey | XKJCHHZQLQNZHY-UHFFFAOYSA-N |
| LogP | 1.15 |
| CAS DataBase Reference | 85-41-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 1J6PQ7YI80 |
| NIST Chemistry Reference | Phthalimide(85-41-6) |
| EPA Substance Registry System | Phthalimide (85-41-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H335-H315 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P | |||||||||
| Risk Statements | 20/21/22-36/37/38-40 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | TI3920000 | |||||||||
| Autoignition Temperature | >500 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29251995 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 10000 mg/kg LD50 dermal Rabbit > 7940 mg/kg | |||||||||
| NFPA 704 |
|
Phthalimide price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.07303 | Phthalimide for synthesis | 85-41-6 | 100g | $30.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.07303 | Phthalimide for synthesis | 85-41-6 | 500g | $65.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.07303 | Phthalimide for synthesis | 85-41-6 | 1kg | $99.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.07303 | Phthalimide for synthesis | 85-41-6 | 50kg | $3240 | 2024-03-01 | Buy |
| Sigma-Aldrich | 240230 | Phthalimide ≥99% | 85-41-6 | 50g | $13.9 | 2024-03-01 | Buy |
Phthalimide Chemical Properties,Uses,Production
Chemical Properties
white to slightly yellowish crystalline flakes
Uses
Phthalimide is a reagent used to transform allyl- and alkyl halides into protected primary amines. Phthalimide analogues have been extensively used in medicinal chemistry owing to their wide spectrum of applications as anti-convulsant, anti-inflammatory, analgesic, hypolipidimic and immunomodulatory activities. Dyes and metabolites, Environmental Testing.
Uses
Phthalimide, C6H4(CO)2NH, is an imide of commercial and industrial importance, forming a number of interesting derivatives. With alcoholic potash, phthalimide forms a potassium derivative, C6H4 (CO)2 NK, which, when reacted with ethyl iodide (or other alkyl halides), yields ethylphthalimide, C6H4(CO)2 N C2H5. The latter product, when hydrolyzed with an acid or alkali, further yields ethylamine [CAS: 75-04-7] C2H7N. Such reaction chains are useful in the preparation of certain primary amines and their derivatives.
Uses
Phthalimide is a reagent used to transform allyl- and alkyl halides into protected primary amines. Phthalimide analogues have been extensively used in medicinal chemistry owing to their wide spectrum of applications as anti-convulsant, anti-inflammatory, analgesic, hypolipidimic and immunomodulatory activities.
Definition
ChEBI: A dicarboximide that is 2,3-dihydro-1H-isoindole substituted by oxo groups at positions 1 and 3.
Synthesis Reference(s)
Journal of the American Chemical Society, 111, p. 3725, 1989 DOI: 10.1021/ja00192a034
The Journal of Organic Chemistry, 45, p. 363, 1980 DOI: 10.1021/jo01290a038
Tetrahedron Letters, 34, p. 6907, 1993 DOI: 10.1016/S0040-4039(00)91827-6
General Description
White to light tan powder. Slightly acidic.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
O-Phthalimide is an imide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). O-Phthalimide forms salts with bases.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition O-Phthalimide emits toxic fumes of nitrogen oxides.
Fire Hazard
Literature sources indicate that O-Phthalimide is combustible.
Safety Profile
Moderately toxic by intraperitoneal route. Mildly toxic by ingestion. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx.
Purification Methods
Crystallise the imide from EtOH (20mL/g) (charcoal), or sublime it. For potassium phthalimide see entry in “Metal-organic Compounds”, Chapter 5. [Beilstein 21/10 V 270.]
Phthalimide Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| shandong exelon biochem co.ld | +86-533-2119995 +8615053330977 | 451614802@QQ.com | China | 28 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12457 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| Springchem New Material Technology Co.,Limited | +86-021-62885108 +8613917661608 | info@spring-chem.com | China | 2068 | 57 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
Related articles
- Preparation method and application of phthalimide
- This article describes a background overview and preparation and use of Phthalimide
- Apr 22,2022
View Lastest Price from Phthalimide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-01-02 | Phthalimide
85-41-6
|
US $100.00-1000.00 / KG | 1KG | 0.99 | 20 tons | Zibo Hangyu Biotechnology Development Co., Ltd | |
 |
2023-11-15 | Phthalimide
85-41-6
|
US $25.00-9.00 / kg | 1kg | 0.99 | 20 tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-09-06 | Phthalimide
85-41-6
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. |
-

- Phthalimide
85-41-6
- US $100.00-1000.00 / KG
- 0.99
- Zibo Hangyu Biotechnology Development Co., Ltd
-

- Phthalimide
85-41-6
- US $25.00-9.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- Phthalimide
85-41-6
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.