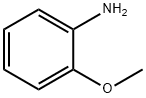
o-Anisidine
- CAS No.
- 90-04-0
- Chemical Name:
- o-Anisidine
- Synonyms
- 2-METHOXYANILINE;ORTHO ANISIDINE;2-ANISIDINE;2-Methoxybenzenamine;2-Methoxy-phenylamine;nsc3122;ai3-08584;O-ANISOLE;2-Anisidin;O-ANISIDINE
- CBNumber:
- CB1286995
- Molecular Formula:
- C7H9NO
- Molecular Weight:
- 123.15
- MDL Number:
- MFCD00007688
- MOL File:
- 90-04-0.mol
- MSDS File:
- SDS
| Melting point | 3-6 °C(lit.) |
|---|---|
| Boiling point | 225 °C(lit.) |
| Density | 1.092 g/mL at 25 °C(lit.) |
| vapor pressure | <0.1 at 25 °C (NIOSH, 1994) |
| refractive index |
n |
| Flash point | 210 °F |
| storage temp. | Refrigerator |
| solubility | 14g/l |
| form | Powder/Solid |
| pka | 4.52(at 25℃) |
| color | Off-white |
| PH | 7.3 (1g/l, H2O, 20℃) |
| Water Solubility | 13 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,667 |
| BRN | 386210 |
| Henry's Law Constant | 1.25 at 25 °C (approximate - calculated from water solubility and vapor pressure) |
| Exposure limits | Potential occupational carcinogen. NIOSH REL: TWA 0.5 mg/m3, IDLH 50 mg/m3; OSHA PEL: TWA 0.5 mg/m3; ACGIH TLV: TWA 0.1 ppm (adopted). |
| Dielectric constant | 5.2300000000000004 |
| Stability | Stable. Incompatible with strong oxidizing agents, acid anhydrides, chloroformates, acids, some plastics, rubber. Air sensitive. |
| InChIKey | VMPITZXILSNTON-UHFFFAOYSA-N |
| LogP | 1.180 |
| CAS DataBase Reference | 90-04-0(CAS DataBase Reference) |
| EWG's Food Scores | 5 |
| FDA UNII | NUX042F201 |
| Proposition 65 List | o-Anisidine |
| IARC | 2A (Vol. Sup 7, 73, 127) |
| NIST Chemistry Reference | Benzenamine, 2-methoxy-(90-04-0) |
| EPA Substance Registry System | o-Anisidine (90-04-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311+H331-H341-H350 | |||||||||
| Precautionary statements | P201-P280-P301+P310+P330-P302+P352+P312-P304+P340+P311 | |||||||||
| Hazard Codes | T,Xi | |||||||||
| Risk Statements | 45-23/24/25-68 | |||||||||
| Safety Statements | 53-45 | |||||||||
| RIDADR | UN 2431 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | BZ5410000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29222200 | |||||||||
| Toxicity | Acute oral LD50 for rats 2,000 mg/kg, wild birds 422 mg/kg, mice 1,400 mg/kg, rabbits 870 mg/kg (quoted, RTECS, 1985). | |||||||||
| IDLA | 50 mg/m3 | |||||||||
| NFPA 704 |
|
o-Anisidine price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A88182 | o-Anisidine ≥99% | 90-04-0 | 5g | $36.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 31597 | o-Anisidine analytical standard | 90-04-0 | 250mg | $46.1 | 2021-12-16 | Buy |
| TCI Chemical | A0486 | o-Anisidine >98.0%(GC)(T) | 90-04-0 | 25mL | $26 | 2024-03-01 | Buy |
| TCI Chemical | A0486 | o-Anisidine >98.0%(GC)(T) | 90-04-0 | 500mL | $120 | 2024-03-01 | Buy |
| Alfa Aesar | A10288 | o-Anisidine, 99% | 90-04-0 | 250g | $41.6 | 2024-03-01 | Buy |
o-Anisidine Chemical Properties,Uses,Production
Description
o-Anisidine was produced commercially in the United States from the 1920s until late 1950s. By 2009, worldwide only six industries manufactured o-anisidine, but none produced hydrochloride salt. o-Anisidine was available from 44 suppliers, including 20 US suppliers, and the hydrochloride salt was available from eight suppliers, including five US suppliers. US imports of o-anisidine and its hydrochloride salt are reported in the category ‘o-anisidines, p-anisidines, and p-phenetidine,’ and US exports are reported in the category ‘anisidines, dianisidines, phenetidines, and their salts.’ From 1989 to 2008, imports ranged from a high of over 4.6 million kg in 1996 to zero in 2007 and 2008, and exports ranged from zero to 262 000 kg. Reports filed under the US Environmental Protection Agency’s (EPA) Toxic Substances Control Act Inventory Up Rule indicated that United States production plus imports of o-anisidine totaled 500 000 lb–1 million lb in 1986, 1990, and 2006; 1 million–10 million lb in 1990 and 1998; and 10 000–500 000 lb in 2002.
Chemical Properties
Anisidine exists as ortho-, meta-, and paraisomers. They have characteristic amine (fishy) odors.
o-Anisidine (or o-methoxyaniline) is an aromatic amine with a methoxyl group ortho to the amino group of aniline. It is a colorless to yellowish, pink, or reddish liquid.o-Anisidine is soluble in water and mineral oils, and miscible with alcohol, benzene, acetone, and diethylether. o-Anisidine hydrochloride, a salt of o-anisidine, is a grayish crystalline solid or powder at room temperature and is soluble in water (NTP, 2011).
Physical properties
Colorless, yellow to reddish liquid with an amine-like odor. Becomes brown on exposure to air.
Uses
In the preparation of azo dyes; corrosion inhibitor; chemical intermediate
Uses
It is primarily used as a chemical intermediate in the production of pigments, dyes, pharmaceuticals, and fragrances. o-Anisidine hydrochloride is used as a chemical intermediate in the production of numerous azo and triphenylmethane dyes and pigments (e.g., C.I. direct red 72, disperse orange 29, direct yellow 44, direct red 24, and acid red 4); in the production of pharmaceuticals, including the expectorant guaiacol; as a corrosion inhibitor for steel; and as an antioxidant for polymercaptan resins.
Uses
Similar to other aromatic amines, o-anisidine may cause methemglobinemia and cancer in humans. It is used mainly as an intermediate for the production of azo dyes and pigments. Other industrial uses of o-anisidine include synthesis of other dyes and pharmaceuticals, as a corrosion inhibitor for steel, and as an antioxidant for polymercaptan resins (IARC, 1999; HSDB, 2012).
Intermediate for azo dyes and for guaiacol.
Definition
ChEBI: O-anisidine is a substituted aniline that is aniline in which the hydrogen ortho to the amino group has been replaced by a methoxy group. It is used as a chemical intermediate in the synthesis of azo pigments and dyes. It has a role as a reagent and a genotoxin. It is a monomethoxybenzene, a substituted aniline and a primary amino compound.
Synthesis Reference(s)
Tetrahedron Letters, 24, p. 4733, 1983 DOI: 10.1016/S0040-4039(00)86242-5
General Description
Clear, yellowish to reddish or brown liquid with an amine (fishy) odor.
Air & Water Reactions
o-Anisidine darkens on exposure to air. Insoluble in water.
Reactivity Profile
o-Anisidine is sensitive to heat. o-Anisidine is also sensitive to exposure to light. o-Anisidine is incompatible with strong oxidizers. o-Anisidine is also incompatible with acids, acid chlorides, acid anhydrides and chloroformates. o-Anisidine will attack some forms of plastics, rubber and coatings. .
Hazard
Strong irritant. Toxic when absorbed through the skin. Possible carcinogen.
Health Hazard
o-Anisidine was carcinogenic in experimental animals.
Fire Hazard
o-Anisidine is combustible.
Safety Profile
Confirmed carcinogen. Moderately toxic by ingestion. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Potential Exposure
Anisidines are used in the manufacture of azo dyes; pharmaceuticals; textile-processing chemicals Incompatibilities: Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine,bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Attacks some coatings and some forms of plastic and rubber.
Carcinogenicity
o-Anisidine is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity in experimental animals.
Environmental Fate
Biological. o-Anisidine should be biodegradable according to OECD guidelines (Brown and Labouerer (1983). Chemical/Physical. At influent concentrations (pH 3.0) of 10, 1.0, 0.1, and 0.01 mg/L, the GAC adsorption capacities were 52, 20, 7.8, and 3.0 mg/g, respectively. At pHs 7 and 9, the GAC adsorption capacities were 110, 50, 23, and 10 mg/g at influent concentrations of 10, 1.0, 0.1, and 0.01 mg/L, respectively (Dobbs and Cohen, 1980).
Shipping
UN2431 Anisidines, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
It is separated from the m-and p-isomers by steam distillation. It is also separated from its usual synthetic precursor o-nitroanisole by dissolving it in dilute HCl (pH <2.0) extracting the nitro impurity with Et2O, adjusting the pH to ~8.0 with NaOH, extracting the amine into Et2O or steam distilling. Extract the distillate with Et2O, dry the extract (Na2SO4), filter, evaporate and fractionate the residual oil. Protect the almost colourless oil from light which turns it yellow in color. [Biggs & Robinson J Chem Soc 3881961, Nodzu et al. Yakugaku Zasshi (J Pharm Soc Japan) 71 713, 715 1951, Beilstein 13 IV 806.]
Toxicity evaluation
It is primarily metabolized by cytochrome P450 isozymes, and it forms two o-anisidine–DNA adducts with DNA. It causes DNA damage by a metabolite in the presence of metals such as Cu(II). It can also cause Cu(II)-mediated oxidative DNA damage.
Waste Disposal
Dissolve in combustible solvent (alcohols, benzene, etc.) and spray solution into furnace equipped with afterburner and scrubber, or burn spill residue on sand and soda ash absorbent in a furnace.
o-Anisidine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of7
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 | admin@firsky-cn.com | China | 436 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
View Lastest Price from o-Anisidine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-10-08 | 2-Anisidine
90-04-0
|
US $30.00 / L | 1L | 99% | 20T | Firsky International Trade (Wuhan) Co., Ltd | |
 |
2023-09-08 | o-Anisidine
90-04-0
|
US $1.00 / kg | 1kg | 99% | 20tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-08-24 | o-Anisidine
90-04-0
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |
-

- 2-Anisidine
90-04-0
- US $30.00 / L
- 99%
- Firsky International Trade (Wuhan) Co., Ltd
-

- o-Anisidine
90-04-0
- US $1.00 / kg
- 99%
- Hebei Yanxi Chemical Co., Ltd.
-

- o-Anisidine
90-04-0
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd