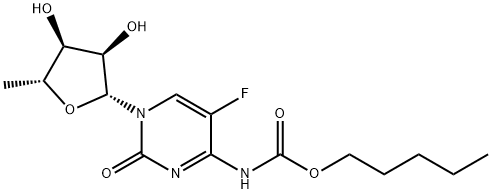
Capecitabine
- CAS No.
- 154361-50-9
- Chemical Name:
- Capecitabine
- Synonyms
- XELODA;Capcitabine;Capecitibine;Capecitabines CAS 154361-50-9;CS-1964;Captabin;RO-9-1978;Ro 09-1978;Chi Pei he;capitabine
- CBNumber:
- CB1324457
- Molecular Formula:
- C15H22FN3O6
- Molecular Weight:
- 359.35
- MDL Number:
- MFCD00930626
- MOL File:
- 154361-50-9.mol
| Melting point | 110-121°C |
|---|---|
| Density | 1.49±0.1 g/cm3(Predicted) |
| Flash point | 87℃ |
| storage temp. | 2-8°C |
| solubility | H2O: soluble10mg/mL, clear (warmed) |
| form | powder |
| pka | 5.41±0.40(Predicted) |
| color | white to beige |
| Merck | 14,1754 |
| InChIKey | GAGWJHPBXLXJQN-UORFTKCHSA-N |
| SMILES | C[C@H]1O[C@@H](N2C=C(F)C(NC(OCCCCC)=O)=NC2=O)[C@H](O)[C@@H]1O |
| CAS DataBase Reference | 154361-50-9(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | capecitabine; Xeloda |
| FDA UNII | 6804DJ8Z9U |
| NCI Drug Dictionary | capecitabine |
| ATC code | L01BC06 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H341-H350-H360FD | |||||||||
| Precautionary statements | P201-P308+P313 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 45-60-61-68 | |||||||||
| Safety Statements | 53-22-36/37-45 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | HA3852500 | |||||||||
| HS Code | 29349990 | |||||||||
| NFPA 704 |
|
Capecitabine price More Price(68)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 1090706 | Capecitabine United States Pharmacopeia (USP) Reference Standard | 154361-50-9 | 200mg | $174.4 | 2024-03-01 | Buy |
| TCI Chemical | C2878 | Capecitabine >98.0%(HPLC)(T) | 154361-50-9 | 1g | $118 | 2024-03-01 | Buy |
| TCI Chemical | C2878 | Capecitabine >98.0%(HPLC)(T) | 154361-50-9 | 5g | $407 | 2024-03-01 | Buy |
| Cayman Chemical | 10487 | Capecitabine ≥98% | 154361-50-9 | 1g | $89 | 2024-03-01 | Buy |
| Cayman Chemical | 10487 | Capecitabine ≥98% | 154361-50-9 | 5g | $371 | 2024-03-01 | Buy |
Capecitabine Chemical Properties,Uses,Production
Indications and Uses
Capecitabine is a new form of oral fluorinated pyrimidine drug. Capecitabine was developed by Roche Pharmaceuticals, and its commercial name is Xeloda. Capecitabine can change in vivo into 5- FU, an anti-metabolizim fluorine pyrimidine deoxynucleoside carbamate drug that targets cancer cells to inhibit cell division and disrupt RNA and protein synthesis. Its effects are significantly tied to the level of TP enzyme expression in neoplastic tissue and to DPD enzyme in vivo expression. It is suitable as further treatment for advanced primary or metastatic breast cancer patients who have not responded to paclitaxel or anthracycline antibiotics. As an anticancer drug, it is mostly used to treat advanced primary or metastatic breast cancer, as well as in treatment for non-small cell lung cancer, pancreatic cancer, bladder cancer, rectal cancer, colon cancer, gastric cancer, and other solid tumors. Many drugs such as taxanes (paclitaxel, docetaxel, mitomycin, cisplatin, etc.) can increase TP enzyme expression in neoplastic tissues and also have curative effects on gastric cancer. When used in combination with Capecitabine, these drugs can also improve Capecitabine’s anticancer abilities and produce synergistic effects.
Clinical trials
Analyses of nearly 400 randomized comparisons of clinical outcomes show that a combination of adriamycin, cisplatin or oxaliplatin with 5-FU, compared to a combination of these drugs with Capecitabine, has a two-fold difference in curative efficacy and lowers toxicty. A large-scale Chinese clinical trial of Capecitabine in combination with DDP in the treatment of stage II gastric cancer also proves its advantages of high efficacy, low toxicity, and affordability.
Drug interactions
Currently, there are no side effects of clinical significance when used in combination with antihistamines, NSAIDs, morphine, paracetamol, aspirin, antiemetic drugs, and H2 receptor antagonist drugs.
Capecitabine’s binding rate with serum protein is relatively low (64%), and its possibility of interacting through substitution with drugs that bind closely with proteins is currently unknown. In external experiments, Capecitabine has not shown any influence on human liver microsomal P450 enzyme.
If any phenytoin and coumarin derivatives anticoagulants are used in combination with Capecitabine, dosages should be lowered.
Adverse effects
Common adverse reactions include nausea, vomiting, oral ulcers, abdominal pain, diarrhea, loss of appetite, and skin changes. There have also been reports of some patients experiencing transient myelosuppression, hair loss, tears, headache, and dizziness.
Warnings and precautions
Capecitabine is a bone marrow inhibitor; a blood exam must be administered before every usage to monitor blood cell and platelet count.
This product poses toxic side effects to the liver, so liver functions must be routinely examined. Additionally, heart functions should also be monitored to prevent irreversible toxic reactions. If venous transfusion of the drug is required, the aforementioned organ functions should be tested for suitability before administration. Capecitabine may cause damage to embryos, thus making it unsuitable for pregnant women. Women using this drug are also not suitable for pregnancy. Even after treatment is ended, this drug may have some impact on fertility.
Description
Capecitabine is a new oral fluoropyrimidine carbamate for patients with advanced neoplastic disease, approved as Xeloda for the treatment of refractory metastatic breast cancer after failure on Paclitaxel and an anthracycline-based chemotherapy regimen ; it is a prodrug of doxifluridine (5-fluorouracil ; 5-FU) activated by a cascade of 3 enzymes concentrated in human liver and cancer tissue, resulting in the selective release of 5-FU at the tumor site and offering a prolonged tumour exposure to 5-FU. Oral Capecitabine passes intact through the intestinal mucosa, is converted first by carboxylesterase to 5'-deoxy-5- fluorocytidine in the liver, then by cytidine deaminase to 5'-deoxy-5-fluorouridine in the liver and tumour tissues and finally by thymidine phosphorylase to 5-FU in tumors. Therefore, Xeloda is much safer and more effective than 5-FU (for example, in the HCT116 human colon cancer and the MX-1 breast cancer xenograft .models).
Chemical Properties
Colourless solid
Originator
Roche (Switzerland)
Uses
An antiproliferative 5-fluorouracil releasing compound
Uses
Capecitabine is an antineoplastic agent. Capecitabine is a prodrug of Doxifluridine (D556750).
Definition
ChEBI: A carbamate ester that is cytidine in which the hydrogen at position 5 is replaced by fluorine and in which the amino group attached to position 4 is converted to its N-(penyloxy)carbonyl derivative. Capecitabine is a antineoplastic agen used in the treatment of cancers.
Manufacturing Process
5-Deoxy-5-fluoro-N4-((n-pentyloxy)carbonyl)cytidine may be prepared
according to US Patent No. 6,114,520.
From 2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-fluorocytidine and n-pentylchloroformate in dichloromethane and pyridine may be obtained 2',3'-
bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-fluoro-N4-((pentyloxy)carbonyl)
cytidine.From 2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-fluoro-N4-
((pentyloxy)carbonyl)cytidineand tetrabutylammonium fluoride in
tetrahydrofuran at room temperature for 2 hours may be prepared the
product which by hydrolyses may be converted to 5-deoxy-5-fluoro-N4-
((pentyloxy)carbonyl)cytidine. Purification of the product may be carried out
by silica gel chromatography (using dichloromethane:methanol = 20:1 as an
eluent).
brand name
Xeloda (Roche).
Therapeutic Function
Antitumor
General Description
The drug is available in a 150- and 500-mg tablets for oraluse. This drug is a fluoropyrimidine carbamate prodrugform of 5-fluorouracil (5-FU). It is used to treat breast cancerand colorectal cancer. The drug is converted to 5-FU bythe enzyme thymidine phosphorylase following esterase activity to hydrolyze the carbamate moiety and deamination.Capecitabine is readily absorbed by the GI tract, and peakplasma levels of 5-FU occur about 2 hours after oral administration.Indications, drug interactions, and toxicities areequivalent to those of 5-FU.
Biochem/physiol Actions
Capecitabine is an anti-cancer drug, a prodrug of doxifluridine, metabolized to 5-fluorouracil at the tumor site. The activation of capecitabine follows a pathway with three enzymatic steps and two intermediary metabolites, 5′-Deoxy-5-fluorocytidine (5′-DFCR) and 5′-Deoxy-5-fluorouridine (5′-DFUR), to form 5-fluorouracil.
Clinical Use
Capecitabine is indicated for use as first-line therapy in patients with colorectal cancer. It also is used alone or in combination with docetaxel in patients with metastatic breast cancer who have experienced disease progression or recurrence after anthracycline therapy. Given b.i.d. in tablet form, the total daily dose is calculated based on patient body surface area and is taken 30 minutes after eating to avoid food-induced decreases in absorption. In addition to bone marrow suppression, nausea, and vomiting, the drug can induce severe diarrhea and a potentially disabling disorder termed “hand-and-foot syndrome” (palmar-plantar erythrodysethesia). Capecitabine inhibits CYP2C9 and, along with competition for serum protein binding sites, results in clinically significant drug–drug interactions with both warfarin and phenytoin.
in vitro
in antiproliferative assays, both ls174t wt and ls174t-c2 cells were more sensitive to capecitabine when cultivated in the same plates as hepg2 hepatoma. in ls174t wt alone and cultivated with hepg2, ic50values of capecitabine were 890 ± 48 and 630 ± 14 μm respectively. the ic50fell from 330 ± 4 down to 89 ± 6 μmin ls174t-c2 subline when cultivated in the same plates as hepatoma cells.in the ls174t-c2 subclone, whereas little cell death occurred in cells exposed to capecitabine, both early and late apoptosis were increased by 244 and 262%, respectively [1]. furthermore, capecitabine induces apoptosis in a fas-dependent manner, and shows a 7-fold higher cytotoxicity and markedly stronger apoptotic potential in thymidine phosphorylase (tp)-transfected ls174t-c2 cells [2].
in vivo
capecitabine was effective in a wider dose range in cxf280, hct116, colo205, and widr human colon cancer xenograft models [2]. in highly metastatic nude mice model, capecitabine inhibited tumor growth and metastatic recurrence after resection of hcc attributed to the high expression of pd-ecgf in tumors [3].
Drug interactions
Potentially hazardous interactions with other drugs
Allopurinol: avoid concomitant use.
Anticoagulants: possibly enhances effect of
coumarins.
Antiepileptics: reported toxicity with fosphenytoin
and phenytoin, due to increased phenytoin levels.
Antipsychotics: avoid with clozapine - increased risk
of agranulocytosis.
Folic acid: toxicity of capecitabine increased - avoid.
Metabolism
Although capecitabine is a carbamylated analogue of cytidine , the drug actually is another 5-fluoro-dUMP prodrug. Given orally, it is extensively metabolized to fluorouracil, which is then converted to the active fluorinated deoxyribonucleotide as previously described. Thymidine phosphorylase, an enzyme involved in this biotransformation, is much more active in tumors than in normal tissue, which improves the tumor-selective generation of fluorouracil. Levels of active drug in the tumor can be up to 3.5-fold higher than in surrounding tissue, leading to a lower incidence of side effects compared to fluorouracil therapy. Because capecitabine is biotransformed to fluorouracil, it follows the same catabolic and elimination pathways reported for 5-fluorouracil.
storage
Store at +4°C
References
[1]. ishikawa t, utoh m, sawada n, et al. tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts[j]. biochemical pharmacology, 1998, 55(7): 1091-1097.
[2]. ishikawa t, utoh m, sawada n, et al. tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts[j]. biochemical pharmacology, 1998, 55(7): 1091-1097.
[3]. zhou j, tang z y, fan j, et al. capecitabine inhibits postoperative recurrence and metastasis after liver cancer resection in nude mice with relation to the expression of platelet-derived endothelial cell growth factor[j]. clinical cancer research, 2003, 9(16): 6030-6037.
Capecitabine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| SHANDONG BOYUAN PHARMACEUTICAL CO., LTD. | +86-0531-69954981 +8615666777973 | dwyane.wang@boyuanpharm.com | China | 211 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | sales@sdperfect.com | China | 294 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 | 2691956269@qq.com | China | 359 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 | sarah@tnjone.com | China | 848 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
Related articles
- Capecitabine for the treatment of pancreatic cancer
- Capecitabine is an oral chemotherapy drug used for colorectal and breast cancer. It has shown promise in treating locally adva....
- Jan 30,2024
- Capecitabine:Synthesis, Mechanism, Pharmacokinetics, Application, Toxicity
- Capecitabine is a novel oral fluoropyrimidine carbamate that is preferentially converted to the cytotoxic moiety fluorouracil ....
- Mar 2,2023
View Lastest Price from Capecitabine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Capecitabine
154361-50-9
|
US $0.00 / KG | 2KG | USP34 | 20tons | Sinoway Industrial co., ltd. | |
 |
2024-04-15 | Capecitabine
154361-50-9
|
US $0.00 / kg | 1kg | 99% | 20tons | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2024-03-13 | Capecitabine
154361-50-9
|
US $0.00-0.00 / KG | 1KG | 99 | 20tons | Shanghai Affida new material science and technology center |
-

- Capecitabine
154361-50-9
- US $0.00 / KG
- USP34
- Sinoway Industrial co., ltd.
-

- Capecitabine
154361-50-9
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- Capecitabine
154361-50-9
- US $0.00-0.00 / KG
- 99
- Shanghai Affida new material science and technology center